
Concept explainers
a)
The thermal efficiency of an ideal diesel cycle using constant specific heats.
a)
Answer to Problem 161RP
The thermal efficiency of ideal diesel cycle is
Explanation of Solution
Draw
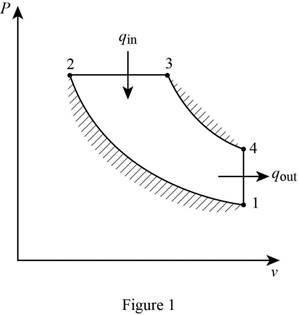
Assuming constant specific heats
Write the temperature and specific volume relation for the isentropic compression process 1-2.
Here, the specific heat ratio is
Write the ideal gas relation for the constant pressure heat addition process 2-3.
For the process 2-3,
Here, temperature at state 3 is
Write the expression of heat input to the cycle
Here, the specific heat at constant pressure is
Write the temperature and specific volume relation for isentropic expansion process 3-4.
Write the expression of heat rejected for constant volume heat rejection process 4-1
Here, specific heat at constant volume is
Write the expression to calculate the net work output of the engine
Write the expression of thermal efficiency of the ideal diesel cycle
Conclusion:
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following properties of air at room temperature.
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the thermal efficiency of an ideal diesel cycle is
b)
The thermal efficiency of ideal diesel cycle using variable specific heats.
b)
Answer to Problem 161RP
The thermal efficiency of an ideal diesel cycle is
Explanation of Solution
Assuming variable specific heats
Write the specific volume and relative specific volume relation for the isentropic compression process 1-2.
Here, the compression ratio is r, relative specific volume at state 1 is
Write the pressure, temperature, and specific volume relation for isentropic compression process 2-3.
For process 2-3,
Write the expression of heat addition for constant pressure heat addition process 2-3
Write the specific volume and relative specific volume relation for the isentropic expansion process 3-4.
Here, relative specific volume at state 4 is
Write the expression of heat rejected for constant volume heat rejection process 4-1
Write the expression of thermal efficiency of an deal diesel cycle
Conclusion:
Refer Table A-17, “Ideal gas properties of air”, obtain the following properties of air at temperature
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Substitute
Refer Table A-17, “Ideal gas properties of air”, obtain the properties of air at
Substitute
Substitute
Thus, the thermal efficiency of an ideal diesel cycle is
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- An HEV has a 24kW battery. How many miles can it go on electricity alone at 40 mph on a flat straight road with no headwind? Assume the rolling resistance factor is 0.018 and the Coefficient of Drag (aerodynamic) is 0.29 the frontal area is 2.25m^2 and the vehicle weighs 1618 kg.arrow_forwardAs shown in the figure below, moist air at T₁ = 36°C, 1 bar, and 35% relative humidity enters a heat exchanger operating at steady state with a volumetric flow rate of 10 m³/min and is cooled at constant pressure to 22°C. Ignoring kinetic and potential energy effects, determine: (a) the dew point temperature at the inlet, in °C. (b) the mass flow rate of moist air at the exit, in kg/min. (c) the relative humidity at the exit. (d) the rate of heat transfer from the moist air stream, in kW. (AV)1, T1 P₁ = 1 bar 11 = 35% 120 T₂=22°C P2 = 1 bararrow_forwardThe inside temperature of a wall in a dwelling is 19°C. If the air in the room is at 21°C, what is the maximum relative humidity, in percent, the air can have before condensation occurs on the wall?arrow_forward
- The inside temperature of a wall in a dwelling is 19°C. If the air in the room is at 21°C, what is the maximum relative humidity, in percent, the air can have before condensation occurs on the wall?arrow_forward###arrow_forwardFind the closed loop transfer function and then plot the step response for diFerentvalues of K in MATLAB. Show step response plot for different values of K. Auto Controls Show solution for transform function and provide matlab code (use k(i) for for loop NO COPIED SOLUTIONSarrow_forward
- This is an old practice exam. The answer is Ta-a = 4.615 MPa max = 14.20 MPa Su = 31.24 MPa Sus = 10.15 MPa but why?arrow_forwardThis is an old practice exam. The answer is dmin = 42.33 mm but how?arrow_forward5.) 12.124* - Block B (WB = 12 lb) rests as shown on the upper surface of wedge A (W₁ = 30 lb). The angle of the slope is 0 = 30°. Neglect friction, and find immediately after the system is released from rest (a) the acceleration of a (a) and (b) the acceleration of B relative to A (a B/A).arrow_forward
- What is the Maximum Bending Moment induced in the following Beam, if? P = 19 KN L = 11 m Ensure that your answer is in kN.m. لا اللهarrow_forwardWhat is the Magnitude of the Maximum Stress in the beam below if? W。 = 6 kN/m L = 9 m Beam width, b = 226 mm Beam Height, h = 273 mm Give your answer in MPa. A 233 B 4|3 Woarrow_forwardWhat is the Reaction Force induced in the following system at point A, if? W = 12 kN/m P = 35 kN L = 11 m Ensure that your answer is in kN. ولها A 4/2 ↓↓ P Barrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





