
Consider a simple ideal Brayton cycle with air as the working fluid. The pressure ratio of the cycle is 6, and the minimum and maximum temperatures are 300 and 1300 K, respectively. Now the pressure ratio is doubled without changing the minimum and maximum temperatures in the cycle. Determine the change in (a) the net work output per unit mass and (b) the thermal efficiency of the cycle as a result of this modification. Assume variable specific heats for air.
a)
The change in net work output per unit mass.
Answer to Problem 164RP
The change in net work output per unit mass is
Explanation of Solution
Draw the
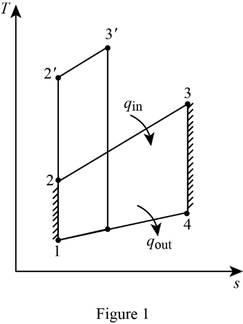
Write the expression for the pressure ratio in terms of relative pressure to calculate the relative pressure at state 2
Here, compressor inlet pressure is
Write the expression for the pressure ratio in terms of relative pressure to calculate the relative pressure at state 4
Here, compressor inlet pressure is
Write the expression for the heat added
Here, specific enthalpy of air from the exit of compressor is
Write the expression for the heat rejected
Here, specific enthalpy of air at the exit of the turbine is
Write the expression for net work done
Write the expression for net work done
Write the expression for the thermal efficiency
Write the expression for the thermal efficiency
Write the expression for change in the net work
Here, net work done for the initial cycle is
Conclusion:
For relative pressure
From the Table A-17, “Ideal-gas properties of air” obtain the following properties at temperature
From the Table A-17, “Ideal-gas properties of air” obtain the following properties at temperature
Substitute
Substitute
From the Table A-17, “Ideal-gas properties of air”.
Obtain the value of enthalpy at state 2
Write the formula of interpolation method of two variables.
Here, the variables denoted by x and y are relative pressure and enthalpy.
Show relative pressure and enthalpy values from the Table A-17.
| Relative pressure | Enthalpy |
| 7.824 | 492.74 |
| 8.316 | ? |
| 8.411 | 503.02 |
Substitute
The value of enthalpy at state 2
Similarly by using interpolation method obtain the value of enthalpy at state 4
Substitute
Substitute
Substitute
Substitute
For relative pressure
Substitute
From the Table A-17, “Ideal-gas properties of air” obtain the values of enthalpy
Substitute
From the Table A-17, “Ideal-gas properties of air” obtain the values of enthalpy
Substitute
Substitute
Substitute
Substitute
Substitute
Thus, the change in net work output per unit mass is
b)
The change in thermal efficiency of the cycle.
Answer to Problem 164RP
The change in thermal efficiency of the cycle is
Explanation of Solution
Write the expression to calculate the change in thermal efficiency
Conclusion:
Substitute
Thus, the change in thermal efficiency of the cycle is
Want to see more full solutions like this?
Chapter 9 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
- A compression ratio of 8 is achieved by using an ideal air-standard Otto cycle engine. The working fluid has a pressure of 100 kPa and a temperature of 27°C at the start of the compression process, and the constant volume heat addition process supplies 800 kJ/kg of heat to the working fluid. What are the (a )the temperature, volume and pressure of the air at the end of each process (in K, m3 and kPa) (b) the net work output/cycle [kJ/kg], and (c) the thermal efficiency of this engine cyclearrow_forwardExhaust gases from the turbine of a simple Brayton cycle are quite hot and may be used for other thermal purposes. One proposed use is generating saturated steam at 110°C from water at 30°C in a boiler. This steam will be distributed to several buildings on a college campus for space heating. A Brayton cycle with a pressure ratio of 6 is to be used for this purpose. Plot the power produced, the flow rate of produced steam, and the maximum cycle temperature as functions of the rate at which heat is added to the cycle. The temperature at the turbine inlet is not to exceed 2000°C.arrow_forwardConsider a simple ideal Brayton cycle with air as the working fluid. The pressure ratio of the cycle is 6, and the minimum and maximum temperatures are 300 and 1300 K, respectively. Now the pressure ratio is doubled without changing the minimum and maximum temperatures in the cycle. Determine the change in the net work output per unit mass.arrow_forward
- Consider an ideal Brayton cycle with air as the working fluid. The pressure ratio of the cycle is 10, mass flow rate is 200 kg/s and the minimum and maximum temperatures are 300 and 1500 K, respectively. Now the pressure ratio is increased 50 percent without changing the minimum and maximum temperatures in the cycle. Assuming constant specific heats, compare both operational conditions in terms power and thermal efficiency. Also show both cycles in T-s diagram together.arrow_forwardThe net work output and the thermal efficiency for the Carnot and the simple ideal Rankine cycles with steam as the working fluid are to be calculated and compared. Steam enters the turbine in both cases at 5 MPa as a saturated vapor, and the condenser pressure is 50 kPa. In the Rankine cycle, the condenser exit state is saturated liquid and in the Carnot cycle, the boiler inlet state is saturated liquid. Draw the T-s diagrams for both cycles.arrow_forwardPravinbhaiarrow_forward
- An ideal Brayton cycle with air as the working fluid has a pressure ratio of 7 and the minimum & maximum temperatures are 300 K and 1300 K, respectively. Now the pressure ratio is doubled without changing the minimum and maximum temperatures in the cycle. Determine the thermal efficiency of the cyclearrow_forwardA simple ideal Brayton cycle operates with air with minimum and maximum temperatures of 27°C and 727°C. It is designed so that the maximum cycle pressure is 2000 kPa and the minimum cycle pressure is 100 kPa. Determine the net work produced per unit mass of air each time this cycle is executed and the cycle’s thermal efficiency. Use constant specific heats at room temperaturearrow_forwardIn an air standard dual cycle two –thirds of the total heat supply occurs at constant volume . The state at the beginning of the compression process is 90kPa and 20°C and the compression ratio is 9. if the total heat supply is2100kJ/kg, Determine the efficiency of the cycle.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





