
A turbojet aircraft is flying with a velocity of 280 m/s at an altitude of 9150 m, where the ambient conditions are 32 kPa and −32°C. The pressure ratio across the compressor is 12, and the temperature at the turbine inlet is 1100 K. Air enters the compressor at a rate of 50 kg/s, and the jet fuel has a heating value of 42,700 kJ/kg. Assuming ideal operation for all components and constant specific heats for air at room temperature, determine (a) the velocity of the exhaust gases, (b) the propulsive power developed, and (c) the rate of fuel consumption.
a)
The velocity of the exhaust gases.
Answer to Problem 133P
The velocity of the exhaust gases is
Explanation of Solution
Draw the
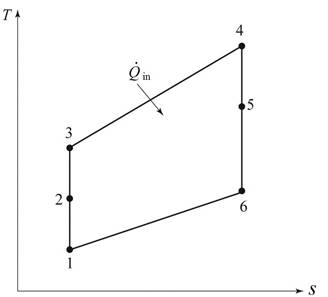
Consider, the pressure is
Consider that the aircraft is stationary, and the velocity of air moving towards the aircraft is
Diffuser (For process 1-2):
Write the expression for the energy balance equation for the diffuser.
Here, the energy entering the system is
Write the temperature and pressure relation for the process 1-2.
Here, the specific heat ratio of air is k.
Compressor (For process 2-3)
Write the pressure relation using the pressure ratio for the process 2-3.
Here, the pressure ratio is
Write the temperature and pressure relation for the process 2-3.
Turbine (For process 4-5)
Write the temperature relation for the compressor and turbine to calculate the temperature at state 5
Nozzle (For process 5-6)
Write the temperature and pressure relation for the isentropic process 4-6.
Write the expression for the energy balance equation for the nozzle.
Conclusion:
From Table A-2a, “Ideal-gas specific heats of various common gases”, obtain the following values of air at room temperature.
The rate of change in the energy of the system
Substitute
Here, the specific heat at constant pressure of air is
Substitute 0 for
Substitute 32 kPa for
Substitute 12 for
Substitute 280.0 K for
Substitute 1,100 K for
For process 5-6 (Nozzle)
Substitute 1100 K for
The rate of change in the energy of the system
Substitute
Substitute 810.5 K for
Thus, the velocity of the exhaust gases is
b)
The propulsive power produced by the turbojet engine.
Answer to Problem 133P
The propulsive power produced by the turbojet engine is
Explanation of Solution
Nozzle (For process 5-6)
Write the expression to calculate the propulsive power produced by the turbojet engine
Here, the velocity of the aircraft is
engine is
Conclusion:
Substitute
Thus, the propulsive power produced by the turbojet engine is
c)
The rate of fuel consumption.
Answer to Problem 133P
The rate of fuel consumption is
Explanation of Solution
Write the expression to calculate the heating value of the fuel for the turbojet engine
Write the expression to calculate the mass flow rate of fuel for the turbojet engine
Here, the calorific value of the fuel is HV.
Conclusion.
Substitute
Substitute
Thus, the rate of fuel consumption is
Want to see more full solutions like this?
Chapter 9 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
- 2. Consider the rod with an elliptical that strain 4 a Cross secton considered in class, Integrate the was displacement displacements, relations to obtain thearrow_forwardPlease answer Oxygen at 300 kPa and 90°C flowing at an average velocity of 3 m/s is expanded in an adiabatic nozzle. What is the maximum velocity of the oxygen at the outlet of this nozzle when the outlet pressure is 60 kPa? Use the table containing the ideal gas specific heats of various common gases. The maximum velocity of the oxygen at the outlet of this nozzle is 532.5 Numeric ResponseEdit Unavailable. 532.5 incorrect.m/s.arrow_forwardA container filled with 70 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Assume the room is at the sea level, well sealed, and heavily insulated. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Determine the amount of heat transfer between the water and the air in the room. The amount of heat transfer between the water and the air in the room is kJ.arrow_forward
- A strain gauge rosette that is attached to the surface of a stressed component gives 3 readings (ɛa = A, b = B, &c = C). If the strain gauge rosette is of the D° type (indicating the angle between each of the gauges), construct a Mohr's Strain Circle overleaf. You should assume that gauge A is aligned along the x-axis. Using the Mohr's Strain Circle calculate the: (i) principal strains (ε1, 2)? (ii) principal angles (1, 2)? You should measure these anticlockwise from the y-axis. (iii) maximum shear strain in the plane (ymax)?arrow_forwardQ1. If the yield stress (σy) of a material is 375MPa, determine whether yield is predicted for the stresses acting on both the elements shown below using: (a) Tresca Criterion (b) Von Mises Criterion P Element A R S Element B Note: your values for P (vertical load on Element A) should be negative (i.e. corresponding to a compressive vertical load).arrow_forwardQ. After a puncture a driver is attempting to remove a wheel nut by applying a force of P KN to one end of a wheel brace as shown in Fig. 1. In cross-section the brace is a hollow steel tube (see section aa) of internal diameter r mm and external diameter q mm. wheel nut n Position S P m r q Section aa Fig, 1 (a) Calculate (i) the twisting moment, (ii) the bending moment, and (iii) the shear force in the brace at position S due to the applied load P. (b) Calculate (i) the shear stress due to twisting, and (ii) the bending stress at position S. Note that the shear force will not produce any shear stress at S. (c) Calculate the maximum shearing stress in the brace at position S using the Maximum Shear Stress Criterion. 2 Mechanics of Materials 2 Tutorials Portfolio: Exercise 5 (d) If the maximum permissible shear stress in the steel is 200 MPa, determine the maximum torque that can be applied by the brace without the risk of failure at S.arrow_forward
- Calculate the first 5 Fourier series coefficients (A0-4 and B1-5 ) for the estimated R wave.arrow_forwardRefrigerant-134a is expanded isentropically from 600 kPa and 70°C at the inlet of a steady-flow turbine to 100 kPa at the outlet. The outlet area is 1 m2, and the inlet area is 0.5 m2. Calculate the inlet and outlet velocities when the mass flow rate is 0.65 kg/s. Use the tables for R-134a. The inlet velocity is m/s. The outlet velocity is m/s.arrow_forwardA container filled with 70 kg of liquid water at 95°C is placed in a 90-m3 room that is initially at 12°C. Thermal equilibrium is established after a while as a result of heat transfer between the water and the air in the room. Assume the room is at the sea level, well sealed, and heavily insulated. NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. Determine the final equilibrium temperature. Use the table containing the ideal gas specific heats of various common gases. The final equilibrium temperature is °C.arrow_forward
- Steam at 100 psia and 650°F is expanded adiabatically in a closed system to 10 psia. Determine the work produced, in Btu/lbm, and the final temperature of steam for an isentropic expansion efficiency of 80 percent. Use steam tables. The work produced is Btu/lbm. The final temperature of steam is °F.arrow_forwardComplet the solution : Vavg Ti Te Ts Q hexp Nuexp htheo Re Nutheo Error (m/s) (*C) (*C) (*C) (W) 2.11 18.8 21.3 45.8 2.61 18.5 20.8 46.3arrow_forwardA 48-kg iron block and a 76-kg copper block, both initially at 80°C, are dropped into a large lake at 15°C. Thermal equilibrium is established after a while as a result of heat transfer between the blocks and the lake water. Determine the total entropy change for this process. The specific heat of iron at room temperature is cp = 0.45 kJ/kg·K. The specific heat of copper at 27°C is cp = 0.386 kJ/kg·K. The total entropy change for this process is kJ/K.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





