
Hydrogen is produced in the steam reforming of propane:
The water-gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen:
The reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125°C, and the products emerge at 800°C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing the exhaust gas from a nearby boiler over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m3/mol C3H8, entering the unit at 1400°C and 1 atm and leaving at 900°C. The unit may be considered adiabatic.
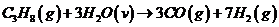
Calculate the molar composition of the product gas, assuming that the heat capacity of the heating gas is 0.040 kJ/(mol·°C).
- Is the reaction process exothermic or endothermic? Explain how you know. Then explain how running the reaction in a reactor-heat exchanger improves the process economy.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ELEM PRINC CHEM (LL) W/EBOOK
Additional Engineering Textbook Solutions
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Degarmo's Materials And Processes In Manufacturing
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
- Metal clusters and catalytic processes.arrow_forwardMetal clusters and catalysis.arrow_forwardQ1: Draw a valid Lewis structures for the following molecules. Include appropriate charges and lone pair electrons. If there is more than one Lewis structure available, draw the best structure. NH3 Sulfate Boron tetrahydride. C3H8 (linear isomer) OCN NO3 CH3CN SO2Cl2 CH3OH2*arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





