
Concept explainers
Ethylbenzene is converted to styrene in the catalytic dehydrogenation reaction C«H10(g) -
A flowchart of a simplified version of the commercial process is shown here.
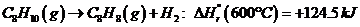
Fresh and recycled liquid ethylbenzene combine and are heated from 25°C to 500°C (A). and the heated ethylbenzene is mixed adiabatically with steam at 700°C (B) to produce the feed to the reactor at 600°C. (The steam suppresses undesired side reactions and removes carbon deposited on the catalyst surface.) A once-through conversion of 35% is achieved in the reactor (C), and the products emerge at 560°C. The product stream is cooled to 25°C (D), condensing essentially all of the water, ethylbenzene, and styrene and allowing hydrogen to pass out as a recoverable by-product of the process.
The water and hydrocarbon liquids are immiscible and are separated in a settling tank decanter (£). The water is vaporized and healed (F) to produce the steam that mixes with the ethylbenzene feed to the reactor. The hydrocarbon stream leaving the decanter is fed to a distillation tower(C) (actually, a series of towers), which separates the mixture into essentially pure styrene and ethylbenzene, each at 25°C after cooling and condensation steps have been carried out. The ethylbenzene is recycled to the reactor preheater, and the styrene is taken off as a product.
- On a basis of 100 kg/h styrene produced, calculate the required fresh ethylbenzene feed rale, the flow rate of recycled ethylbenzene, and the circulation rate of water, all in mol/h. (Assume P = 1 atm.)
- Calculate the required rates of heat input or withdrawal in kJ/h for the ethylbenzene preheater (A), steam generator (F), and reactor (C).
Physical Property Data
Learn your wayIncludes step-by-step video

Chapter 9 Solutions
ELEM PRIN OF CHEMICAL PROC(LL)+NEXTGEN
Additional Engineering Textbook Solutions
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Electric Circuits. (11th Edition)
Database Concepts (8th Edition)
Modern Database Management
Mechanics of Materials (10th Edition)
- Can you provide me the answer of these pleasearrow_forwardA constant-volume process involving 2.0 moles of diatomic ideal gas, for which cV = (5/2)R, is at an initial state of 111 kPa and 277 K and is then heated reversibly to 356 K. Calculate the P2 (kPa), dU (kJ), heat transfer q (kJ mol–1), and w (kJ mol–1).arrow_forwardProblem 1 Marks: 60 Section: 1a): 30 marks, Section 1b):30 marks A laboratory scale fluidized bed is considered for studying a catalytic ozone decomposition. a) It is requested to derive model equations under the following assumptions: ■ Operation of the catalytic reactor under steady state conditions, There is no influence of thermal ozone decomposition reactions. The fluidized bed includes bubbles and dense phase. □ The dense phase can be simulated using a CSTR The fluidized bed bubbles contain catalyst particles and can be simulated as a DSTR (batch). □ The jets contain particles and can be simulated with a PFR. The influence of the freeboard has to be considered using a PFR model. The available catalytic reaction rate model is r (moles/gcat.s)= -k CA b) Same than on a) under unsteady state conditions, using an absorbable and reactive tracer. Note: A step-by step derivation of the model equations is required here. A quick answer will not do. Problem 2 Marks: 40 Section 2a: 30 marks,…arrow_forward
- Penicillin process Penicillium chrysogenum is used to produce penicillin in a 90,000-litre fermenter. The volumetric rate of oxygen uptake by the cells ranges from 0.45 to 0.85 mmol l^−1 min^−1 depending on time during the culture. Power input by stirring is 2.9 W l^−1. Estimate the cooling requirements.arrow_forwardProduction of bakers’ yeast Bakers’ yeast is produced in a 50,000-litre fermenter under aerobic conditions. The carbon substrate is sucrose; ammonia is provided as the nitrogen source. The average biomass composition is CH_1.83O_0.55N_0.17 with 5% ash. Under conditions supporting efficient growth, biomass is the only major product and the biomass yield from sucrose is 0.5 g g^−1. If the specific growth rate is 0.45 h^−1, estimate the rate of heat removal required to maintain constant temperature in the fermenter when the yeast concentration is 10 g l^−1.arrow_forwardSensible energy change Calculate the enthalpy change associated with the following processes:(a) m-Cresol is heated from 25°C to 100°C(b) Ethylene glycol is cooled from 20°C to 10°C(c) Succinic acid is heated from 15°C to 120°C(d) Air is cooled from 150°C to 65°Carrow_forward
- ▼ Enzyme conversion An immobilised enzyme process is used in an ice-cream factory to hydrolyse lactose (C12H22O11) to glucose (C6H12O6) and galactose (C6H1206): C12H22O11 + H2O →→ C6H12O + C6H12O6 Gel beads containing ß-galactosidase are packed into a column reactor; 2500 kg of lactose enters the reactor per day as a 10% solution in water at 25°C. The reactor operates at steady state and 32°C; all of the lactose is converted. Because the heat of reaction for enzyme conversions is not as great as for cell culture, sensible heat changes and heats of mixing cannot be ignored. Lactose Water Ah (kJ gmol¹) C, (cal g¹ ºC-¹) Ahm (kcal gmol¹) 3.7 -5652.5 0.30 1.0 Glucose -2805.0 0.30 5.6 Galactose -2805.7 0.30 5.6 (a) What is the standard heat of reaction for this enzyme conversion? (b) Estimate the heating or cooling requirements for this process. State explicitly whether heating or cooling is needed.arrow_forwardBacterial production of alginate Azotobacter vinelandii is investigated for production of alginate from sucrose. In a continuous fermenter at 28°C with ammonia as nitrogen source, the yield of alginate was found to be 4 g g^−1 oxygen consumed. It is planned to produce alginate at a rate of 5 kg h^−1. Since the viscosity of alginate in aqueous solution is considerable, energy input due to mixing the broth cannot be neglected. The fermenter is equipped with a flat-blade disc turbine; at a satisfactory mixing speed and air flow rate, the power requirements are estimated at 1.5 kW. Calculate the cooling requirements.arrow_forwardPreheating nutrient medium Steam is used to heat nutrient medium in a continuous-flow process. Saturated steam at 150°C enters a coil on the outside of the heating vessel and is completely condensed. Liquid medium enters the vessel at 15°C and leaves at 44°C. Heat losses from the jacket to the surroundings are estimated as 0.22 kW. If the flow rate of medium is 3250 kg h^−1 and its heat capacity is 0.9 cal g^−1 °C^−1, how much steam is required?arrow_forward
- Q3] Determine the optimal operating conditions (XA, t, and CR) in a mixed flow reactor to maximize the concentration of R (CR) in the effluent, where an aqueous feed A with an initial concentration of CA0-40 mol/m³ enters the reactor, undergoes decomposition, and exits as a mixture containing A, R, and S. K₁ AR, FR = k₁C, k₁ = 0.4 m³/(mol min) SA AS, rs = k₂CA, k₂ = 2(min), CA0 = 40 mol/m³arrow_forwardConsider the parallel decomposition of A of different orders with FR = 1, rs = 2CA and IT = C. Determine the maximum concentration of desired product obtainable in mixed flow reactor and plug flow reactor. (1) R is desired product and CA0 = 2. (2) S is desired product and CA0 = 4. R S Tarrow_forward1. Copper is known to be toxic to fish, and in particular, the free ion Cu²+ species typically shows greatest toxicity. a. Calculate the speciation of Cu(II) for freshwater at a pH value of 8.3 as the hardness increases from 20 (soft) to 100 (moderately hard) to 200 (very hard) mg/L as CaCO3. Assume that the total divalent Cu(II) concentration is 10 µg/L. b. Based on your findings in part (a), do you think it is appropriate to set a single regulatory limit or should it depend on variables such as pH and hardness? Explain your answer.arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





