
What are the common and systematic names of the following ethers?
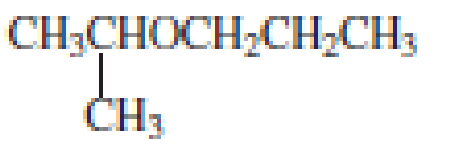
- a. CH3CH2CH2CH2OCH2CH3
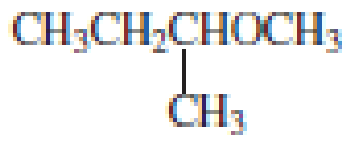
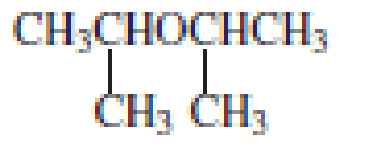
(a)
Interpretation:
The systematic name and the common name of the given ether have to be given.
Concept introduction:
IUPAC naming of Ether compounds:
An ether group consists of oxygen atom attached between two carbon chains. The shorter of two chains becomes the first part of the name with ‘ane’ changes to ‘oxy’ and the longer alkane becomes the suffix of the name of the ether.
When the Oxygen is not at the terminal position of main chain of alkane, then the shorter alkyl group and the ether group together are treated as a side chain and prefixed with its position of bonding on the main chain.
Common name for ether compounds:
- The name of the groups attached to the oxygen has to be given in alphabetical order followed by the word ether.
Explanation of Solution
The structure of the compound is given below:
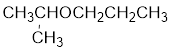
From the structure of the compound, it is understood that the compound is an ether with three carbons in the parent chain. Hence, the parent chain will be propane. The numbering of the longest chain will be in such a way to get the carbon atom attached to the oxygen the possible lowest number.
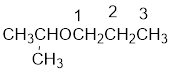
The shorter alkyl group attached to the oxygen is the isopropyl group.
Therefore, systematic name of the compound is given below:
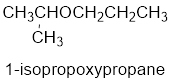
The common name of the compound can be identified by naming the alkyl groups attached to the oxygen followed by the word ether. In the given compound, one iso propyl group and one propyl group is attached to oxygen.
Therefore, common name of the compound is given below:
Propyl isopropyl ether
(b)
Interpretation:
The systematic name and the common name of the given ether has to be given.
Concept introduction:
IUPAC naming of Ether compounds:
An ether group consists of oxygen atom attached between two carbon chains. The shorter of two chains becomes the first part of the name with ‘ane’ changes to ‘oxy’ and the longer alkane becomes the suffix of the name of the ether.
When the Oxygen is not at the terminal position of main chain of alkane, then the shorter alkyl group and the ether group together are treated as a side chain and prefixed with its position of bonding on the main chain.
Common name for ether compounds:
- The name of the groups attached to the oxygen has to be given in alphabetical order followed by the word ether.
Explanation of Solution
The structure of the compound is given below:
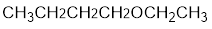
From the structure of the compound, it is understood that the compound is an ether with four carbons in the parent chain. Thus the parent chain will be butane. The numbering of the longest chain will be in such a way to get the carbon atom attached to the oxygen the possible lowest number.
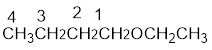
The shorter alkyl group attached to the oxygen that is in the first carbon is the ethyl group and therefore, the prefix will be “ethoxy”.
Therefore, the systematic name of the compound is given below:
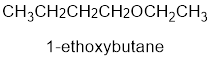
The common name of the compound can be identified by naming the alkyl groups attached to the oxygen followed by the word ether. In the given compound, one ethyl group and one butyl group is attached to oxygen.
Therefore, common name of the compound is given below:
butyl ethyl ether.
(c)
Interpretation:
The systematic name and the common name of the given ether has to be given.
Concept introduction:
IUPAC naming of Ether compounds:
An ether group consists of oxygen atom attached between two carbon chains. The shorter of two chains becomes the first part of the name with ‘ane’ changes to ‘oxy’ and the longer alkane becomes the suffix of the name of the ether.
When the Oxygen is not at the terminal position of main chain of alkane, then the shorter alkyl group and the ether group together are treated as a side chain and prefixed with its position of bonding on the main chain.
Common name for ether compounds:
- The name of the groups attached to the oxygen has to be given in alphabetical order followed by the word ether.
Explanation of Solution
The structure of the compound is given below:
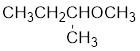
From the structure of the compound, it is understood that the compound is an ether with four carbons in the parent chain. Thus the parent chain will be butane. The numbering of the longest chain will be in such a way to get the carbon atom attached to the oxygen the possible lowest number.
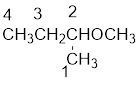
The oxygen is attached to the second carbon.
The shorter alkyl group attached to the oxygen is the methyl group and therefore, the prefix will be “methoxy”.
Therefore, the systematic name of the compound is given below:
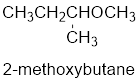
The common name of the compound can be identified by naming the alkyl groups attached to the oxygen followed by the word ether. In the given compound, one sec-butyl group and one methyl group is attached to oxygen.
Therefore, common name of the compound is given below:
Sec-butyl methyl ether.
(d)
Interpretation:
The systematic name and the common name of the given ether has to be given.
Concept introduction:
IUPAC naming of Ether compounds:
An ether group consists of oxygen atom attached between two carbon chains. The shorter of two chains becomes the first part of the name with ‘ane’ changes to ‘oxy’ and the longer alkane becomes the suffix of the name of the ether.
When the Oxygen is not at the terminal position of main chain of alkane, then the shorter alkyl group and the ether group together are treated as a side chain and prefixed with its position of bonding on the main chain.
Common name for ether compounds:
- The name of the groups attached to the oxygen has to be given in alphabetical order followed by the word ether.
Explanation of Solution
The structure of the compound is given below:
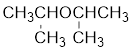
From the structure of the compound, it is understood that the compound is an ether with three carbons in the parent chain. Hence, the parent chain will be propane.
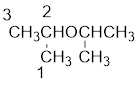
The shorter alkyl group attached to the oxygen that is in the second carbon is the isopropyl group and therefore, the prefix of the name will be “isopropoxy”.
Therefore, systematic name of the compound is given below:
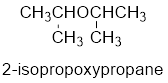
The common name of the compound can be identified by naming the alkyl groups attached to the oxygen followed by the word ether. In the given compound, diisopropyl groups are attached to oxygen.
Therefore, common name of the compound is given below:
disopropyl ether.
Want to see more full solutions like this?
Chapter 9 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
- 93 = Volume 93 = 5.32× 10 3 -23 ст a √ 1073 5.32× 10 3 cm³arrow_forwardASP.....arrow_forwardQuestion 7 (10 points) Identify the carboxylic acid present in each of the following items and draw their structures: Food Vinegar Oranges Yogurt Sour Milk Pickles Acid Structure Paragraph ✓ BI UAE 0118 + v Task: 1. Identify the carboxylic acid 2. Provide Name 3. Draw structure 4. Take a picture of your table and insert Add a File Record Audio Record Video 11.arrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Molecule 1 Molecule 2 IZ IN Molecule 4 Molecule 5 ZI none of the above ☐ Molecule 3 Х IN www Molecule 6 NH Garrow_forwardHighlight each chiral center in the following molecule. If there are none, then check the box under the drawing area. There are no chiral centers. Cl Cl Highlightarrow_forwardA student proposes the following two-step synthesis of an ether from an alcohol A: 1. strong base A 2. R Is the student's proposed synthesis likely to work? If you said the proposed synthesis would work, enter the chemical formula or common abbreviation for an appropriate strong base to use in Step 1: If you said the synthesis would work, draw the structure of an alcohol A, and the structure of the additional reagent R needed in Step 2, in the drawing area below. If there's more than one reasonable choice for a good reaction yield, you can draw any of them. ☐ Click and drag to start drawing a structure. Yes No ロ→ロ 0|0 G Х D : ☐ பarrow_forward
- टे Predict the major products of this organic reaction. Be sure to use wedge and dash bonds when necessary, for example to distinguish between different major products. ☐ ☐ : ☐ + NaOH HO 2 Click and drag to start drawing a structure.arrow_forwardShown below are five NMR spectra for five different C6H10O2 compounds. For each spectrum, draw the structure of the compound, and assign the spectrum by labeling H's in your structure (or in a second drawing of the structure) with the chemical shifts of the corresponding signals (which can be estimated to nearest 0.1 ppm). IR information is also provided. As a reminder, a peak near 1700 cm-1 is consistent with the presence of a carbonyl (C=O), and a peak near 3300 cm-1 is consistent with the presence of an O–H. Extra information: For C6H10O2 , there must be either 2 double bonds, or 1 triple bond, or two rings to account for the unsaturation. There is no two rings for this problem. A strong band was observed in the IR at 1717 cm-1arrow_forwardPredict the major products of the organic reaction below. : ☐ + Х ك OH 1. NaH 2. CH₂Br Click and drag to start drawing a structure.arrow_forward
- NG NC 15Show all the steps you would use to synthesize the following products shown below using benzene and any organic reagent 4 carbons or less as your starting material in addition to any inorganic reagents that you have learned. NO 2 NC SO3H NO2 OHarrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardShow work...don't give Ai generated solutionarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning


