
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
3rd Edition
ISBN: 9780134255644
Author: Bruice, Paula Yurkanis
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 49P
The following reaction takes place several times faster than the reaction of 2-chlorobutance with HO-:
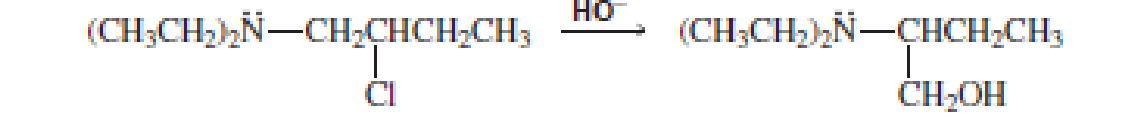
- a. Eplain the enhanced reaction rate.
- b. Explain why the OH group in the product is not bonded to the carbon that was bonded to the Cl group in the reactant.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In addition to the separation techniques used in this lab (magnetism, evaporation, and filtering), there are other commonly used separation techniques. Some of these techniques are:Distillation – this process is used to separate components that have significantly different boiling points. The solution is heated and the lower boiling point substance is vaporized first. The vapor can be collected and condensed and the component recovered as a pure liquid. If the temperature of the mixture is then raised, the next higher boiling component will come off and be collected. Eventually only non-volatile components will be left in the original solution.Centrifugation – a centrifuge will separate mixtures based on their mass. The mixture is placed in a centrifuge tube which is then spun at a high speed. Heavier components will settle at the bottom of the tube while lighter components will be at the top. This is the technique used to separate red blood cells from blood plasma.Sieving – this is…
Briefly describe a eutectic system.
13.53 Draw all stereoisomers formed when each compound is treated with HBr in the presence of peroxides.
a.
b.
C.
Chapter 9 Solutions
Essential Organic Chemistry Study Guide & Solution Manual, Books a la Carte Edition
Ch. 9.1 - Draw the structures of straight-chain alcohols...Ch. 9.1 - Prob. 2PCh. 9.1 - Prob. 3PCh. 9.2 - Why are NH3 and CH3NH2 no longer nucleophiles when...Ch. 9.2 - Prob. 5PCh. 9.2 - The observed relative reactivities of primary,...Ch. 9.4 - Which of the following alcohols would dehydrate...Ch. 9.4 - Prob. 10PCh. 9.4 - Prob. 11PCh. 9.4 - Prob. 12P
Ch. 9.4 - Prob. 13PCh. 9.5 - What product will be obtained from the reaction of...Ch. 9.5 - Prob. 15PCh. 9.6 - a. What is each ethers systematic name? 1....Ch. 9.8 - Draw the structure of the following: a....Ch. 9.8 - Prob. 20PCh. 9.8 - Would you expect the reactivity of a five-membered...Ch. 9.9 - Explain why the two arene oxides in Problem 22...Ch. 9.9 - Which compound is more likely to be...Ch. 9.11 - The following three nitrogen mustards were studied...Ch. 9 - What are the common and systematic names of the...Ch. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Prob. 30PCh. 9 - Prob. 31PCh. 9 - What is the major product obtained from the...Ch. 9 - Draw structures for the following: a....Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Ethylene oxide reacts readily with HO.because of...Ch. 9 - Propose a mechanism for each of the following...Ch. 9 - Which of the following ethers would be obtained in...Ch. 9 - Show how each of the following syntheses could be...Ch. 9 - Prob. 42PCh. 9 - Prob. 43PCh. 9 - Prob. 44PCh. 9 - Propose a mechanism for each of the following...Ch. 9 - a. Propose a mechanism for the following reaction:...Ch. 9 - Three arene oxides can be obtained from...Ch. 9 - Prob. 48PCh. 9 - The following reaction takes place several times...Ch. 9 - Show how each of the following compounds could be...Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - Propose a mechanism for the following reaction:Ch. 9 - What alkenes would you expect to be obtained from...Ch. 9 - Triethylenemelamine (TEM) is an antitumor agent....Ch. 9 - When a diol that has OH groups on adjacent carbons...Ch. 9 - What product is obtained when...Ch. 9 - Prob. 58P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardNonearrow_forwardman Campus Depa (a) Draw the three products (constitutional isomers) obtained when 2-methyl-3-hexene reacts with water and a trace of H2SO4. Hint: one product forms as the result of a 1,2-hydride shift. (1.5 pts) This is the acid-catalyzed alkene hydration reaction.arrow_forward
- (6 pts - 2 pts each part) Although we focused our discussion on hydrogen light emission, all elements have distinctive emission spectra. Sodium (Na) is famous for its spectrum being dominated by two yellow emission lines at 589.0 and 589.6 nm, respectively. These lines result from electrons relaxing to the 3s subshell. a. What is the photon energy (in J) for one of these emission lines? Show your work. b. To what electronic transition in hydrogen is this photon energy closest to? Justify your answer-you shouldn't need to do numerical calculations. c. Consider the 3s subshell energy for Na - use 0 eV as the reference point for n=∞. What is the energy of the subshell that the electron relaxes from? Choose the same emission line that you did for part (a) and show your work.arrow_forwardNonearrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception to the general ionization energy (IE) trend. For the two elements involved, answer the following questions. Be sure to cite sources for all physical data that you use. a. (2 pts) Identify the two elements and write their electronic configurations. b. (2 pts) Based on their configurations, propose a reason for the IE trend exception. c. (5 pts) Calculate effective nuclear charges for the last electron in each element and the Allred-Rochow electronegativity values for the two elements. Can any of these values explain the IE trend exception? Explain how (not) - include a description of how IE relates to electronegativity.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License