
Principles of Chemistry: A Molecular Approach (3rd Edition)
3rd Edition
ISBN: 9780321971944
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 26E
Use formal charge to determine which of the two Lewis structures shown here is better.
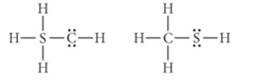
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw two different Lewis structures for ClO3-, one which all atoms follow the octet rule and one where the chlorine has an expanded octet. Determine the formal charge on all atoms for each structure. Which do you think is the better Lewis structure? Explain your reasoning.
A resonance hybrid is a structure that can be depicted by more than one valid Lewis structure.
part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons.
part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons.
part3: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all nonzero formal charges and all nonbonding electrons.
Draw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- :
a) The leftmost bond (between N and N) is a single bond.b) The rightmost bond (between N and N) is a single bond.c) The formal charge on the leftmost (N) atom is -1.d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4.e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.
Chapter 9 Solutions
Principles of Chemistry: A Molecular Approach (3rd Edition)
Ch. 9 - Prob. 9.1PCh. 9 - Arrange the compounds in order or increasing...Ch. 9 - Prob. 9.2MPCh. 9 - Prob. 9.3PCh. 9 - Prob. 9.4PCh. 9 - Prob. 9.5PCh. 9 - Prob. 9.6PCh. 9 - Prob. 9.7PCh. 9 - Prob. 9.8PCh. 9 - Prob. 9.8MP
Ch. 9 - Prob. 9.9PCh. 9 - Write the Lewis structure for XeF4.Ch. 9 - Prob. 9.10MPCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.11MPCh. 9 - Prob. 1SAQCh. 9 - Prob. 2SAQCh. 9 - Prob. 3SAQCh. 9 - Prob. 4SAQCh. 9 - Prob. 5SAQCh. 9 - Prob. 6SAQCh. 9 - Prob. 7SAQCh. 9 - Prob. 8SAQCh. 9 - Prob. 9SAQCh. 9 - Prob. 10SAQCh. 9 - Prob. 11SAQCh. 9 - Prob. 12SAQCh. 9 - Use formal charge to choose the best Lewis...Ch. 9 - Use bond energies to determine Hrxnfor this...Ch. 9 - Prob. 15SAQCh. 9 - Prob. 1ECh. 9 - Prob. 2ECh. 9 - Prob. 3ECh. 9 - Prob. 4ECh. 9 - Prob. 5ECh. 9 - Prob. 6ECh. 9 - Prob. 7ECh. 9 - Prob. 8ECh. 9 - Explain the trend in the lattice energies of the...Ch. 9 - Prob. 10ECh. 9 - The lattice enerqy of CsF is -744 kJ/mol, whereas...Ch. 9 - Prob. 12ECh. 9 - Prob. 13ECh. 9 - Prob. 14ECh. 9 - Prob. 15ECh. 9 - Prob. 16ECh. 9 - Prob. 17ECh. 9 - Prob. 18ECh. 9 - Draw the Lewis structure for CO with an arrow...Ch. 9 - Prob. 20ECh. 9 - Prob. 21ECh. 9 - Prob. 22ECh. 9 - Prob. 23ECh. 9 -
24. Write the Lewis structure that obeys the...Ch. 9 - Prob. 25ECh. 9 - Use formal charge to determine which of the two...Ch. 9 - Prob. 27ECh. 9 - Prob. 28ECh. 9 - Prob. 29ECh. 9 - Prob. 30ECh. 9 - Prob. 31ECh. 9 - What are the formal charges of the atoms shown in...Ch. 9 - Write the Lewis structure for each molecule. a....Ch. 9 - Prob. 34ECh. 9 - Prob. 35ECh. 9 - Prob. 36ECh. 9 - Prob. 37ECh. 9 - Prob. 38ECh. 9 - Consider these three compounds: HCCH, H2CCH2...Ch. 9 - Prob. 40ECh. 9 - Prob. 41ECh. 9 - Prob. 42ECh. 9 - Prob. 43ECh. 9 - Prob. 44ECh. 9 - Prob. 45ECh. 9 - Prob. 46ECh. 9 - Prob. 47ECh. 9 - Amino acids are the building blocks of proteins....Ch. 9 - Prob. 49ECh. 9 - Prob. 50ECh. 9 - Prob. 51ECh. 9 - Prob. 52ECh. 9 - Prob. 53ECh. 9 - Prob. 54ECh. 9 - Prob. 55ECh. 9 - Prob. 56ECh. 9 - Prob. 57ECh. 9 - Prob. 58ECh. 9 - Prob. 59ECh. 9 - Prob. 60ECh. 9 - Prob. 61ECh. 9 - Prob. 62ECh. 9 - Prob. 63ECh. 9 - Calculate Hrxn for the combustion of octane...Ch. 9 - 65. Draw the Lewis structure for each compound.
a....Ch. 9 - Prob. 66ECh. 9 - Prob. 67ECh. 9 - Prob. 68ECh. 9 - Prob. 69ECh. 9 - Prob. 70ECh. 9 - Prob. 71ECh. 9 - Prob. 72ECh. 9 - Prob. 73ECh. 9 - Prob. 74ECh. 9 - Prob. 75ECh. 9 - Prob. 76ECh. 9 - Prob. 77ECh. 9 - Prob. 78ECh. 9 - Prob. 79ECh. 9 - Prob. 80ECh. 9 - Prob. 81ECh. 9 - Prob. 82ECh. 9 - Prob. 83E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A complete Lewis structure must show all nonzero formal charges. Complete each of thefollowing Lewis structures by adding any missing formal charges.arrow_forwardDraw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- : a) The leftmost bond (between N and N) is a single bond. b) The rightmost bond (between N and N) is a single bond. c) The formal charge on the leftmost (N) atom is -1. d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4. e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of +1. Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forward
- Draw three resonance structures for CS,. This species has its three atoms bonded sequentially in the following fashion: S-C-S. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Select the choices from below which make the statements true about this (most important) resonance structure. (a) The leftmost bond (between S and C) is a single v bond. (b) The rightmost bond (between C and S) is a single v bond. (c) The formal charge on the leftmost (S) atom is -Select-v (d) The formal charge on the central (C) atom is -Select---v (e) The formal charge on the rightmost (S) atom is Select-v (f) The number of nonbonding pairs (lone pairs) of electrons in the leftmost (S) atom is Select-v pairs. (g) The number of nonbonding (lone) pairs of electrons in the rightmost (S) atom is -Select-- v pairs.arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of zero. Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forwardAn incompicie Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of -1. N-C-C-H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. H N=c=c-Harrow_forward
- Match each molecule with the formal charge of the element in bold.If several resonance structures are possible, consider only one in which all atoms obey the octet rule. NOClO3 (middle O)CO2arrow_forwardIn the POF, molecule, the P atom is the central atom. Draw a Lewis diagram of POF, for which all formal charges are equal to zero. How many double bonds are there in the structure that you have drawn? number of double bonds = Draw a Lewis diagram in which the octet rule is satisfied on all atoms. What are the formal charges on the following atoms in the structure that you have drawn? P Based on formal charge, which is the best Lewis structure for the molecule?arrow_forwardConsider the molecule with the formula below, where X is the only central atom (all other atoms are directly bonded to X). Draw the most important Lewis structure for the molecule that follows the octet rule using the molecular charge and formal charges listed below. Determine the identity of X and the number of single and double bonds in your structure. Molecule formula: HXO2 Molecular charge: -1 Total number of valence electrons: 18 Formal charge on X: 0 The central atom (X) is: Click for List The molecule's number of single bonds is: The molecule's number of double bonds is: Click for List Click for Listarrow_forward
- Choose the best Lewis structure for OCN. (It will help to work out the missing formal charges for the atoms in these different structures) [Image description: Lewis structure A has a C atom singly bound to a O atom and a N atom. There are three electron pairs on O and three electron pairs on N. Lewis structure B has a C atom singly bound to a O atom and triply bound to a N atom. There are three electron pairs on O and one electron pair on N. Lewis structure C has a C atom triply bound to a O atom and singly bound to a N atom. There is one electron pair on O and three electron pairs on N. Lewis structure D has a C atom doubly bound to a O atom and a N atom. There are two electron pairs on O and two electron pairs on N.] :0-c-N: :0-c=N: :0=C-N: o=c=N: A B Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a b В C C d Darrow_forwardDraw Lewis structures of the following. Select the structure(s) that do NOT obey the octet rule. (for molecules with resonance, consider the best structure by formal charge) H2SO4 CH3- AlCl3 NF3 PH3arrow_forwardDraw a Lewis structure of your choice that has at least three equally reasonable resonance structures. You must show your work as to how the structure was drawn including showing valence electrons, any structures you draw that don’t work and require you to show a multiple bond, and formal charges on each atom that has a formal charge. For example, Nitrate ion has three structures (but you can’t draw this one). All three structures have formal charges and no one structure is preferred based on formal charge. Carbon dioxide would not be a valid choice. While it does have three structures, the structure that has two double bonds is the preferred structure while the structures with triple bonds are not equivalent to the double bonded version as they have multiple formal charges present.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY