
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 15CI
Tamiflu (oseltamivir), C16H28N2O4, is an antiviral drug used to treat influenza. The preparation of Tamiflu begins with the extraction of shikimic acid from the seedpods of star anise. From 2.6 g of star anise,0.13 g of shikimic acid can be obtained and used to produce one capsule containing 75 mg of Tamiflu.The usual adult dosage for treatment of influenza is two capsule of Tamiflu daily for 5 days. (7.1, 7.2)
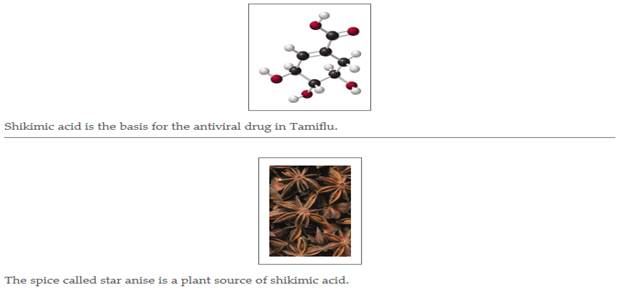
- If black spheres are carbon atoms, white spheres are hydrogen atoms, and red spheres are oxygen atoms, what is the formula of shikimic acid?
- What is the moral mass of shikimic acid?
- How many moles of shikimic acid are contained in 130 g of shikimic acid?
- How many capsules containing 75 mg of Tamiflu could be produced from 155 g of star anise?
- What is the moral mass of Tamiflu?
- How many kilograms of Tamiflu would be needed to treat all the people in a city with a population of 500 000 if each person consumes two Tamiflu capsules a day for 5 days?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How many arrangements are there of 15 indistinguishable lattice gas particles distributed on:
a.V = 15 sites
b.V = 16 sites
c.V = 20 sites
For which element is the 3d subshell higher in energy than that 4s subshell?
Group of answer choices
Zr
Ca
V
Ni
ii) Molecular ion peak
:the peak corresponding to the intact molecule (with a positive charge)
What would the base peak and Molecular ion peaks when isobutane is subjected
to Mass spectrometry? Draw the structures and write the molecular weights of
the fragments.
Circle most stable cation
a) tert-butyl cation
b) Isopropyl cation c) Ethyl cation. d) Methyl cation
6. What does a loss of 15 represent in Mass spectrum?
a fragment of the molecule with a mass of 15 atomic mass units has been lost during
the ionization Process
7. Write the isotopes and their % abundance of isotopes of
i) Cl
Chapter 9 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 9.1 - Prob. 9.1QAPCh. 9.1 - Prob. 9.2QAPCh. 9.1 - Describe the formation of an aqueous KI solution,...Ch. 9.1 - Describe the formation of an aqueous LiBr...Ch. 9.1 - Prob. 9.5QAPCh. 9.1 - Prob. 9.6QAPCh. 9.2 - Prob. 9.7QAPCh. 9.2 - NaOHis a strong electrolyte, and CH3OH is a...Ch. 9.2 - Write a balanced equation for the dissociation of...Ch. 9.2 - Write the balanced equation for the dissociation...
Ch. 9.2 - Indicate whether aqueous solutions of each of the...Ch. 9.2 - Prob. 9.12QAPCh. 9.2 - Prob. 9.13QAPCh. 9.2 - Prob. 9.14QAPCh. 9.2 - Calculate the number of equivalents in each of the...Ch. 9.2 - Calculate the number of equivalents in each of the...Ch. 9.2 - Prob. 9.17QAPCh. 9.2 - Prob. 9.18QAPCh. 9.2 - Prob. 9.19QAPCh. 9.2 - Prob. 9.20QAPCh. 9.3 - Prob. 9.21QAPCh. 9.3 - Prob. 9.22QAPCh. 9.3 - Determine whether each of the following solutions...Ch. 9.3 - Determine whether each of the following solutions...Ch. 9.3 - A solution containing 80. g of KClin 200 g of H2O...Ch. 9.3 - A solution containing 80. g of NaNO3 in 75 g of...Ch. 9.3 - Explain the following observations More sugar...Ch. 9.3 - Explain the following observations: And open can...Ch. 9.3 - Prob. 9.29QAPCh. 9.3 - Prob. 9.30QAPCh. 9.4 - What is the difference between a 5.0% (m/m)...Ch. 9.4 - What is the difference between a 10.0% (v/v)...Ch. 9.4 - Calculate the mass percent (m/m) for the solute in...Ch. 9.4 - Calculate the mass percent (m/m) for the solute in...Ch. 9.4 - Calculate the mass/volume(m/v) percent for the...Ch. 9.4 - Calculate the mass/volume (m/v) percent for the...Ch. 9.4 - Calculate the grams or milliliters of solute...Ch. 9.4 - Calculate the grams or ml of solute needed to...Ch. 9.4 - A mouthwash contains 22.5% (v/v) alcohol.If the...Ch. 9.4 - A bottle of champagne is 11% (v/v) alcohol. If...Ch. 9.4 - A patient received 100 mL of a 20.0% (m/v)...Ch. 9.4 - A patient received 250 mL of a 4.0% (m/v) amino...Ch. 9.4 - A patient needs 100. g of glucose in the next 12...Ch. 9.4 - A patient received 2.0 g of NaCl in 8 h. How many...Ch. 9.4 - Prob. 9.45QAPCh. 9.4 - For each of the following solutions, calculate...Ch. 9.4 - Prob. 9.47QAPCh. 9.4 - Prob. 9.48QAPCh. 9.4 - Calculate the gram of solely needed to prepare...Ch. 9.4 - Calculate the gram of solute needed to prepare...Ch. 9.4 - For each of the following solutions, calculate...Ch. 9.4 - Prob. 9.52QAPCh. 9.5 - Prob. 9.53QAPCh. 9.5 - Prob. 9.54QAPCh. 9.5 - Prob. 9.55QAPCh. 9.5 - Prob. 9.56QAPCh. 9.5 - Determine the final volume, in milliliters, of...Ch. 9.5 - Prob. 9.58QAPCh. 9.5 - Prob. 9.59QAPCh. 9.5 - Prob. 9.60QAPCh. 9.6 - Prob. 9.61QAPCh. 9.6 - Identify each of the following as characteristics...Ch. 9.6 - A 10% (m/v) starch solution is separated form a 1%...Ch. 9.6 - A 0.1% (m/v) albumin solution is separated form a...Ch. 9.6 - Indicate the compartment (A or B) that will...Ch. 9.6 - Indicate the compartment (A or B) that will...Ch. 9.6 - Will a red blood cell undergo creation, hemolysis,...Ch. 9.6 - Will a red blood cell undergo creation, hemolysis,...Ch. 9.6 - Each of the following mixtures is placed in a...Ch. 9.6 - Prob. 9.70QAPCh. 9 - Prob. 9.71UTCCh. 9 - Prob. 9.72UTCCh. 9 - Prob. 9.73UTCCh. 9 - Whydo lettuces leaves in a salad with after a...Ch. 9 - Prob. 9.75UTCCh. 9 - Prob. 9.76UTCCh. 9 - Prob. 9.77UTCCh. 9 - Prob. 9.78UTCCh. 9 - Prob. 9.79AQAPCh. 9 - Prob. 9.80AQAPCh. 9 - Prob. 9.81AQAPCh. 9 - Prob. 9.82AQAPCh. 9 - Prob. 9.83AQAPCh. 9 - Prob. 9.84AQAPCh. 9 - Prob. 9.85AQAPCh. 9 - Prob. 9.86AQAPCh. 9 - Prob. 9.87AQAPCh. 9 - Prob. 9.88AQAPCh. 9 - Prob. 9.89AQAPCh. 9 - Prob. 9.90AQAPCh. 9 - Prob. 9.91AQAPCh. 9 - Prob. 9.92AQAPCh. 9 - Prob. 9.93AQAPCh. 9 - Prob. 9.94AQAPCh. 9 - Prob. 9.95AQAPCh. 9 - Prob. 9.96AQAPCh. 9 - Calculate the final concentration of the solution...Ch. 9 - Calculate the final concentration of the solution...Ch. 9 - Prob. 9.99AQAPCh. 9 - Prob. 9.100AQAPCh. 9 - Prob. 9.101AQAPCh. 9 - Prob. 9.102AQAPCh. 9 - Prob. 9.103AQAPCh. 9 - Prob. 9.104AQAPCh. 9 - Prob. 9.105CQCh. 9 - 9.114. In a laboratory experiment, a 15.0-sample...Ch. 9 - Prob. 9.107CQCh. 9 - Prob. 9.108CQCh. 9 - Prob. 9.109CQCh. 9 - Prob. 9.110CQCh. 9 - Prob. 9.111CQCh. 9 - Prob. 9.112CQCh. 9 - Prob. 13CICh. 9 - The active ingredient in Turns is calcium...Ch. 9 - Tamiflu (oseltamivir), C16H28N2O4, is an antiviral...Ch. 9 - The compound butyric acid gives rancid butter its...Ch. 9 - Methane is a major component of purified natural...Ch. 9 - Automobile exhaust is a major cause of air...Ch. 9 - Bleach is often added to a wash to remove stains...Ch. 9 - Prob. 20CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose a number and match the atomic number to your element on the periodic table. For your element, write each of these features on a side of your figure. 1. Element Name and symbol 2. Family and group 3. What is it used for? 4. Sketch the Valence electron orbital 5. What ions formed. What is it's block on the periodic table. 6. Common compounds 7. Atomic number 8. Mass number 9. Number of neutrons- (show calculations) 10. Sketch the spectral display of the element 11.Properties 12. Electron configuration 13. Submit a video of a 3-meter toss in slow-moarrow_forward[In this question, there are multiple answers to type in a "fill-in-the-blank" fashion - in each case, type in a whole number.] Consider using Slater's Rules to calculate the shielding factor (S) for the last electron in silicon (Si). There will be electrons with a 0.35 S-multiplier, electrons with a 0.85 S-multiplier, and electrons with a 1.00 S-multiplier.arrow_forwardProvide the unknown for the given data.arrow_forward
- Draw the Lewis structures of two methanol (CH3OH) molecules and depict hydrogenbonding between them with dashed lines. Show all lone pairs. Provide a thorough analysis to apply concept idea into other problems.arrow_forwardSteps and explanation please.arrow_forwardHow could you distinguish between each pair of compounds below using IR? For each pair citeone bond and it’s frequency that you could use to distinguish between them. Please provide thorough analysis to apply into further problems.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY