
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
12th Edition
ISBN: 9780321908445
Author: Karen C. Timberlake
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9.6, Problem 9.65QAP
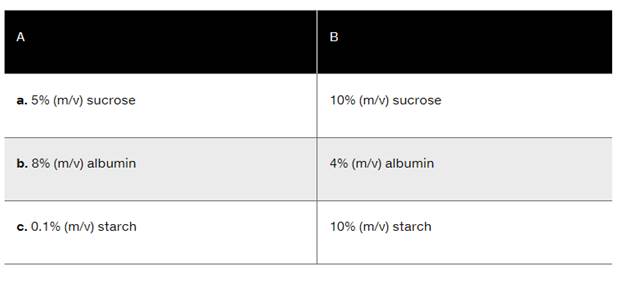
Indicate the compartment (A or B) that will increase in volume for each of the following of the solutions separated by a semipermeable membrane:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
32S
16
Enter your answers numerically separated by a comma.
Np. Nn =
跖
ΟΙ ΑΣΦ
Submit
Request Answer
?
protons, neutrons
2.
Which dimethylcyclohexane compounds shown below exhibit symmetry and therefore
are not chiral and would not rotate plane polarized light.
1
CH3
CH
CH3
CH3
2
3
CH3
Don't used hand raiting
Chapter 9 Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Ch. 9.1 - Prob. 9.1QAPCh. 9.1 - Prob. 9.2QAPCh. 9.1 - Describe the formation of an aqueous KI solution,...Ch. 9.1 - Describe the formation of an aqueous LiBr...Ch. 9.1 - Prob. 9.5QAPCh. 9.1 - Prob. 9.6QAPCh. 9.2 - Prob. 9.7QAPCh. 9.2 - NaOHis a strong electrolyte, and CH3OH is a...Ch. 9.2 - Write a balanced equation for the dissociation of...Ch. 9.2 - Write the balanced equation for the dissociation...
Ch. 9.2 - Indicate whether aqueous solutions of each of the...Ch. 9.2 - Prob. 9.12QAPCh. 9.2 - Prob. 9.13QAPCh. 9.2 - Prob. 9.14QAPCh. 9.2 - Calculate the number of equivalents in each of the...Ch. 9.2 - Calculate the number of equivalents in each of the...Ch. 9.2 - Prob. 9.17QAPCh. 9.2 - Prob. 9.18QAPCh. 9.2 - Prob. 9.19QAPCh. 9.2 - Prob. 9.20QAPCh. 9.3 - Prob. 9.21QAPCh. 9.3 - Prob. 9.22QAPCh. 9.3 - Determine whether each of the following solutions...Ch. 9.3 - Determine whether each of the following solutions...Ch. 9.3 - A solution containing 80. g of KClin 200 g of H2O...Ch. 9.3 - A solution containing 80. g of NaNO3 in 75 g of...Ch. 9.3 - Explain the following observations More sugar...Ch. 9.3 - Explain the following observations: And open can...Ch. 9.3 - Prob. 9.29QAPCh. 9.3 - Prob. 9.30QAPCh. 9.4 - What is the difference between a 5.0% (m/m)...Ch. 9.4 - What is the difference between a 10.0% (v/v)...Ch. 9.4 - Calculate the mass percent (m/m) for the solute in...Ch. 9.4 - Calculate the mass percent (m/m) for the solute in...Ch. 9.4 - Calculate the mass/volume(m/v) percent for the...Ch. 9.4 - Calculate the mass/volume (m/v) percent for the...Ch. 9.4 - Calculate the grams or milliliters of solute...Ch. 9.4 - Calculate the grams or ml of solute needed to...Ch. 9.4 - A mouthwash contains 22.5% (v/v) alcohol.If the...Ch. 9.4 - A bottle of champagne is 11% (v/v) alcohol. If...Ch. 9.4 - A patient received 100 mL of a 20.0% (m/v)...Ch. 9.4 - A patient received 250 mL of a 4.0% (m/v) amino...Ch. 9.4 - A patient needs 100. g of glucose in the next 12...Ch. 9.4 - A patient received 2.0 g of NaCl in 8 h. How many...Ch. 9.4 - Prob. 9.45QAPCh. 9.4 - For each of the following solutions, calculate...Ch. 9.4 - Prob. 9.47QAPCh. 9.4 - Prob. 9.48QAPCh. 9.4 - Calculate the gram of solely needed to prepare...Ch. 9.4 - Calculate the gram of solute needed to prepare...Ch. 9.4 - For each of the following solutions, calculate...Ch. 9.4 - Prob. 9.52QAPCh. 9.5 - Prob. 9.53QAPCh. 9.5 - Prob. 9.54QAPCh. 9.5 - Prob. 9.55QAPCh. 9.5 - Prob. 9.56QAPCh. 9.5 - Determine the final volume, in milliliters, of...Ch. 9.5 - Prob. 9.58QAPCh. 9.5 - Prob. 9.59QAPCh. 9.5 - Prob. 9.60QAPCh. 9.6 - Prob. 9.61QAPCh. 9.6 - Identify each of the following as characteristics...Ch. 9.6 - A 10% (m/v) starch solution is separated form a 1%...Ch. 9.6 - A 0.1% (m/v) albumin solution is separated form a...Ch. 9.6 - Indicate the compartment (A or B) that will...Ch. 9.6 - Indicate the compartment (A or B) that will...Ch. 9.6 - Will a red blood cell undergo creation, hemolysis,...Ch. 9.6 - Will a red blood cell undergo creation, hemolysis,...Ch. 9.6 - Each of the following mixtures is placed in a...Ch. 9.6 - Prob. 9.70QAPCh. 9 - Prob. 9.71UTCCh. 9 - Prob. 9.72UTCCh. 9 - Prob. 9.73UTCCh. 9 - Whydo lettuces leaves in a salad with after a...Ch. 9 - Prob. 9.75UTCCh. 9 - Prob. 9.76UTCCh. 9 - Prob. 9.77UTCCh. 9 - Prob. 9.78UTCCh. 9 - Prob. 9.79AQAPCh. 9 - Prob. 9.80AQAPCh. 9 - Prob. 9.81AQAPCh. 9 - Prob. 9.82AQAPCh. 9 - Prob. 9.83AQAPCh. 9 - Prob. 9.84AQAPCh. 9 - Prob. 9.85AQAPCh. 9 - Prob. 9.86AQAPCh. 9 - Prob. 9.87AQAPCh. 9 - Prob. 9.88AQAPCh. 9 - Prob. 9.89AQAPCh. 9 - Prob. 9.90AQAPCh. 9 - Prob. 9.91AQAPCh. 9 - Prob. 9.92AQAPCh. 9 - Prob. 9.93AQAPCh. 9 - Prob. 9.94AQAPCh. 9 - Prob. 9.95AQAPCh. 9 - Prob. 9.96AQAPCh. 9 - Calculate the final concentration of the solution...Ch. 9 - Calculate the final concentration of the solution...Ch. 9 - Prob. 9.99AQAPCh. 9 - Prob. 9.100AQAPCh. 9 - Prob. 9.101AQAPCh. 9 - Prob. 9.102AQAPCh. 9 - Prob. 9.103AQAPCh. 9 - Prob. 9.104AQAPCh. 9 - Prob. 9.105CQCh. 9 - 9.114. In a laboratory experiment, a 15.0-sample...Ch. 9 - Prob. 9.107CQCh. 9 - Prob. 9.108CQCh. 9 - Prob. 9.109CQCh. 9 - Prob. 9.110CQCh. 9 - Prob. 9.111CQCh. 9 - Prob. 9.112CQCh. 9 - Prob. 13CICh. 9 - The active ingredient in Turns is calcium...Ch. 9 - Tamiflu (oseltamivir), C16H28N2O4, is an antiviral...Ch. 9 - The compound butyric acid gives rancid butter its...Ch. 9 - Methane is a major component of purified natural...Ch. 9 - Automobile exhaust is a major cause of air...Ch. 9 - Bleach is often added to a wash to remove stains...Ch. 9 - Prob. 20CI
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Can you please explain why the answer is structures 2 and 3? Please include a detailed explanation and show how the synthesis can be done with those two structures.arrow_forwardCan you please explain why the correct answer to this question is option 2? I am having trouble understanding how and why. Please provide a detailed explanation and a drawing of how the diene and dienophile would create the product in the question.arrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Base your explanation off of the rules for aromaticity and well as the principles of the Huckel rule of aromaticity. Please give a detailed explanation of what Hucekl's rule is.arrow_forward
- Can you please explain why the answer is B and not A? I chose A because I thought the thermodynamic product was a 1,4-addition. Please give a detailed explanation to this problem and include a drawing of how the reaction works.arrow_forwardLabel the diagram according to the components and processes of an alkaline batteryarrow_forwardCan you please explain why the answer to the question is option 4? Please include the aromaticity rules as well as Huckel's rule. Please label molecules 1, 2, 3, and 5 with their respective labels of aromatic or nonaromatic and why.arrow_forward
- Don't used hand raitingarrow_forwardCan you please explain why the correct answer is molecules 2 and 4? Please provide a detailed explanation as well as the two molecules drawn showing what and where it is conjugated.arrow_forwardCan you please explain why the correct answer is (2E, 4Z, 6Z)-2,4,6-Nonatriene? Please include a detailed explanation and a drawing of the structure, with the corresponding parts of the answer labeled. I'm confused why 6 is Z and why it is Nonatriene.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax
Solutions: Crash Course Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=9h2f1Bjr0p4;License: Standard YouTube License, CC-BY