
a)
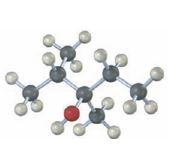
Interpretation:
The structures of
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration methods which method can be used to prepare the alcohol from alkenes.
b)
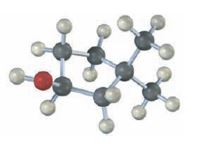
Interpretation:
The structures of alkenes that would yield the alcohol shown on hydration are to be given. Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol also has to be stated.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol.
Trending nowThis is a popular solution!

Chapter 8 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- (a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forwardChoose the right answerarrow_forward
- 8. What is the major product of the following reaction? KMnO4 b a TOH OH OH C d OH "OH HO OH OHarrow_forwardChoose the right answerarrow_forward3. Draw ALL THE POSSBILE PRODUCTS AND THE MECHANISMS WITH ALL RESONANCE STRUCTURES. Explain using the resonance structures why the major product(s) are formed over the minor product(s). H₂SO4, HONO CHarrow_forward
- 7. Provide the product(s), starting material(s) and/or condition(s) required for the No mechanisms required. below reaction HO + H-I CI FO Br2, FeBr3 O I-Oarrow_forward6. Design the most efficient synthesis of the following product starting from phenot Provide the reaction conditions for each step (more than one step is required) and explain the selectivity of each reaction. NO MECHANISMS ARE REQUIRED. OH step(s) CIarrow_forwardWhat is the skeletal structure of the product of the following organic reaction?arrow_forward
- If a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forwardWhat is the major organic product of the following nucleophilic acyl substitution reaction of an acid chloride below?arrow_forwardWould the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



