
Concept explainers
Name the following
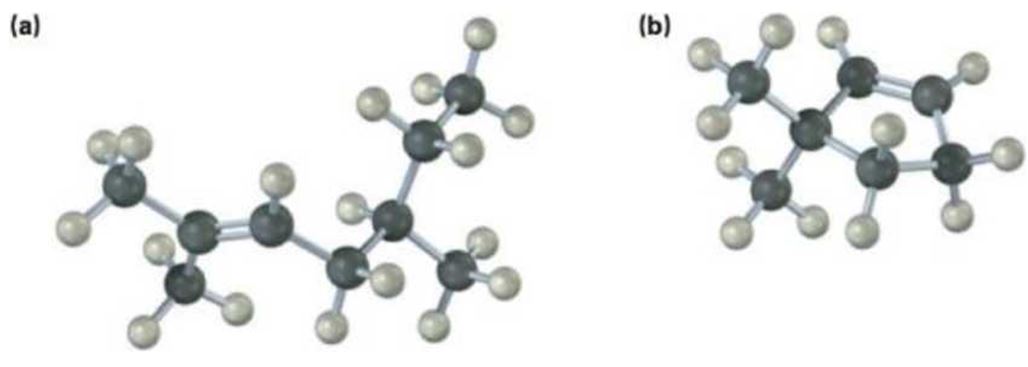
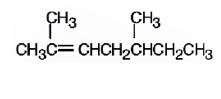
a)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if the alkene the double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
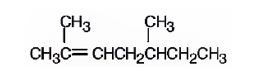
The name of the alkene shown is 2,5-dimethyl-2-heptene.
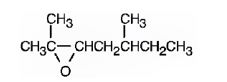
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
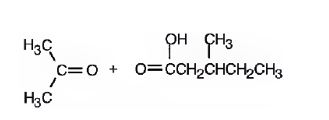
The products formed when it reacts with O3, followed by Zn in acetic acid are
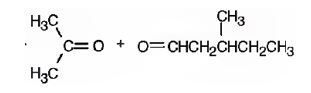
Explanation of Solution
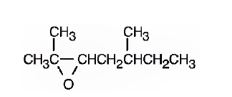
2,5-dimethyl-2-heptene has a double bond between C2&C3. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C2&C3 to give an epoxide.
In 2,5-dimethyl-2-heptene, C2 has no hydrogens attached. So it is oxidized to a ketone.C3 has one hydrogen and is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C2&C3 in 2,5-dimethyl-2-heptene is cleaved and each carbon gets attached to an oxygen atom to give a ketone and aldehyde as products.
The name of the alkene shown is 2,5-dimethyl-2-heptene is
The product formed when it reacts with meta-chloroperoxybenzoic acid is
The products formed when it reacts with KMnO4 in aqueous acid are
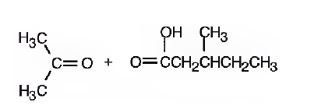
The products formed when it reacts with O3, followed by Zn in acetic acid are
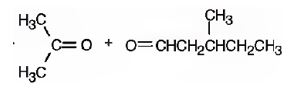
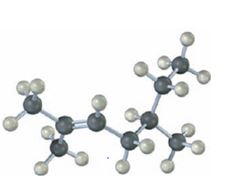
b)
Interpretation:
The name of the alkene shown to be given. The products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid, are to be given.
Concept introduction:
When alkenes react with meta-chloroperoxybenzoic acid, oxygen atom adds to the double bond to give epoxides.
Upon treatment with KMnO4 in aqueous acid, if in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to a carboxylic acid.
Upon treatment with O3, followed by Zn in acetic acid the double bonded is cleaved and each carbon in the double bond gets attached to an oxygen atom to give carbonyl compounds as products. If in the alkene double bonded carbon is di-substituted and has no hydrogen it is converted in to a ketone and if the double bonded carbon is mono-substituted and has one hydrogen it is oxidized to an aldehyde.
To give:
The name of the alkene shown and the products of its reaction with 1) meta-chloroperoxybenzoic acid, 2) KMnO4 in aqueous acid and 3) O3, followed by Zn in acetic acid.
Answer to Problem 22VC
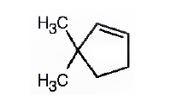
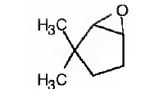
The name of the alkene shown is 3,3-dimethylcyclopentene.
The product formed when it reacts with meta-chloroperoxybenzoic acid is
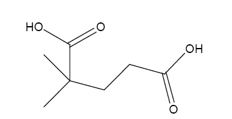
The products formed when it reacts with KMnO4 in aqueous acid are
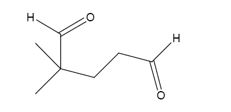
The products formed when it reacts with O3, followed by Zn in acetic acid are
Explanation of Solution
3,3-dimethylcyclopentene has a double bond between C1&C2. When treated with meta-chloroperoxybenzoic acid, oxygen atom adds to both C1&C2 to give an epoxide.
In 3,3-dimethylcyclopentene both C1&C2 have one hydrogen attached to them. So they are oxidized to carboxylic acids when treated with KMnO4.
Upon treatment with O3, followed by Zn in acetic acid the double bond between C1&C2 in 3,3-dimethylcyclopentene is cleaved and each carbon with one hydrogen gets attached to an oxygen atom to yield a dialdehyde as product.
The name of the alkene shown is 3,3-dimethylcyclopentene.

The products formed when it reacts with meta-chloroperoxybenzoic acid are

The products formed when it reacts with KMnO4 in aqueous acid are

The products formed when it reacts with O3, followed by Zn in acetic acid are

Want to see more full solutions like this?
Chapter 8 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- What the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forwardOf the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forward
- please answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forwardDraw the mechanism to make the alcohol 1-hexanol. Please use arrows.arrow_forward
- Answer the followings: 1-What is the difference(s) between DNA and RNA: a- Structure: b- Function: c- Types: 2-What is the meaning of: a- Replication b- Transcription c- Translation 3- Show the base pair connection (hydrogen bond) in DNA and RNAarrow_forwardWhy does the anhydride react with the OH on the benzene rather than the OH on the carboxy group?arrow_forwardAnswer the followings: 1- What is the IP for a amino acid? Give example. 2- What are the types of amino acids? 3- What are the structures of protein? 4- The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N- terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin? 5. MATCH a term from the list below to each definition. Place the letter of the term in the blank to the left of the definition. a. Ligases b. Fibrous proteins c. Conjugated protein d. Hydrolases a. b. C. e. Simple protein f. Globular proteins g. Lyases h. Transferases Proteins that are tough and insoluble in water. Enzymes that catalyze the breaking away of a small molecule such as from a substrate. Enzymes that catalyze the bonding together of two substrates.arrow_forward
- Answer the followings (Four): 1-What is the difference(s) between FOUR: a. Glyceride and phosphoglyceride. b. Wax and fat. c. Soap and fatty acid. d. HDL and LDL cholesterol e. Phospho lipids and sphingosine. 2-What are the types of lipids? 3-What are the main lipid components of membrane structures? 4-How could lipids play important rules as signaling molecules and building units? 5. The Structure variety of Lipids makes them to play significant rules in our body. Conclude briefly on this statement.arrow_forwardHO IV но. = HO но. HO. HO но. зад надо What is the product of the following reaction?arrow_forwardDraw the mechanism to make the alcohol 2-hexanol.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

