
Thermodynamics: An Engineering Approach
9th Edition
ISBN: 9781259822674
Author: Yunus A. Cengel Dr., Michael A. Boles
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.8, Problem 67P
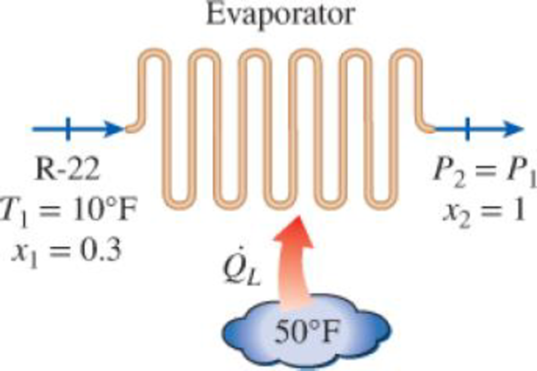
Refrigerant-22 absorbs heat from a cooled space at 50°F as it flows through an evaporator of a refrigeration system. R-22 enters the evaporator at 10°F at a rate of 0.08 lbm/s with a quality of 0.3 and leaves as a saturated vapor at the same pressure. Determine (a) the rate of cooling provided, in Btu/h, (b) the rate of exergy destruction in the evaporator, and (c) the second-law efficiency of the evaporator. Take T0 = 77°F. The properties of R-22 at the inlet and exit of the evaporator are: h1 = 107.5 Btu/lbm, s1 = 0.2851 Btu/lbm·R, h2 = 172.1 Btu/lbm, s2 = 0.4225 Btu/lbm·R.
FIGURE P8–67E
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
P₂
7+1
*
P₁
ART
2 P (P₁ - P₂- Zgp)
21 / Prove that :-
m²
a
cda
A₂
==
*
Cde
actual
mip
Solution
Q1/ Show that (actual
02/ A simple iet
==
Cda
Cdf
х
Af
2/Y
-
Y+1/Y
2P(P1-P2-zxgxpr)
5. Determine the transfer function of G(s) = 01(s)/T₁(s) and 02(s)/T₁ for the mechanical system
shown in Figure Q5. (Hints: assume zero initial condition)
T₁(t) 01(t)
102(1)
Ол
N1
D1
D2
No. 1790220000
N2
Figure Q5
K2
Chapter 8 Solutions
Thermodynamics: An Engineering Approach
Ch. 8.8 - What final state will maximize the work output of...Ch. 8.8 - Is the exergy of a system different in different...Ch. 8.8 - Under what conditions does the reversible work...Ch. 8.8 - How does useful work differ from actual work? For...Ch. 8.8 - How does reversible work differ from useful work?Ch. 8.8 - Is a process during which no entropy is generated...Ch. 8.8 - Consider an environment of zero absolute pressure...Ch. 8.8 - It is well known that the actual work between the...Ch. 8.8 - Consider two geothermal wells whose energy...Ch. 8.8 - Consider two systems that are at the same pressure...
Ch. 8.8 - Prob. 11PCh. 8.8 - Does a power plant that has a higher thermal...Ch. 8.8 - Prob. 13PCh. 8.8 - Saturated steam is generated in a boiler by...Ch. 8.8 - One method of meeting the extra electric power...Ch. 8.8 - A heat engine that receives heat from a furnace at...Ch. 8.8 - Consider a thermal energy reservoir at 1500 K that...Ch. 8.8 - A heat engine receives heat from a source at 1100...Ch. 8.8 - A heat engine that rejects waste heat to a sink at...Ch. 8.8 - A geothermal power plant uses geothermal liquid...Ch. 8.8 - A house that is losing heat at a rate of 35,000...Ch. 8.8 - A freezer is maintained at 20F by removing heat...Ch. 8.8 - Prob. 24PCh. 8.8 - Prob. 25PCh. 8.8 - Prob. 26PCh. 8.8 - Can a system have a higher second-law efficiency...Ch. 8.8 - A mass of 8 kg of helium undergoes a process from...Ch. 8.8 - Which is a more valuable resource for work...Ch. 8.8 - Which has the capability to produce the most work...Ch. 8.8 - The radiator of a steam heating system has a...Ch. 8.8 - A well-insulated rigid tank contains 6 lbm of a...Ch. 8.8 - A pistoncylinder device contains 8 kg of...Ch. 8.8 - Prob. 35PCh. 8.8 - Prob. 36PCh. 8.8 - Prob. 37PCh. 8.8 - A pistoncylinder device initially contains 2 L of...Ch. 8.8 - A 0.8-m3 insulated rigid tank contains 1.54 kg of...Ch. 8.8 - An insulated pistoncylinder device initially...Ch. 8.8 - Prob. 41PCh. 8.8 - An insulated rigid tank is divided into two equal...Ch. 8.8 - A 50-kg iron block and a 20-kg copper block, both...Ch. 8.8 - Prob. 45PCh. 8.8 - Prob. 46PCh. 8.8 - Prob. 47PCh. 8.8 - A pistoncylinder device initially contains 1.4 kg...Ch. 8.8 - Prob. 49PCh. 8.8 - Prob. 50PCh. 8.8 - Prob. 51PCh. 8.8 - Air enters a nozzle steadily at 200 kPa and 65C...Ch. 8.8 - Prob. 54PCh. 8.8 - Prob. 55PCh. 8.8 - Argon gas enters an adiabatic compressor at 120...Ch. 8.8 - Prob. 57PCh. 8.8 - Prob. 58PCh. 8.8 - The adiabatic compressor of a refrigeration system...Ch. 8.8 - Refrigerant-134a at 140 kPa and 10C is compressed...Ch. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Combustion gases enter a gas turbine at 900C, 800...Ch. 8.8 - Steam enters a turbine at 9 MPa, 600C, and 60 m/s...Ch. 8.8 - Refrigerant-134a is condensed in a refrigeration...Ch. 8.8 - Prob. 66PCh. 8.8 - Refrigerant-22 absorbs heat from a cooled space at...Ch. 8.8 - Prob. 68PCh. 8.8 - Prob. 69PCh. 8.8 - Air enters a compressor at ambient conditions of...Ch. 8.8 - Hot combustion gases enter the nozzle of a...Ch. 8.8 - Prob. 72PCh. 8.8 - A 0.6-m3 rigid tank is filled with saturated...Ch. 8.8 - Prob. 74PCh. 8.8 - Prob. 75PCh. 8.8 - An insulated vertical pistoncylinder device...Ch. 8.8 - Liquid water at 200 kPa and 15C is heated in a...Ch. 8.8 - Prob. 78PCh. 8.8 - Prob. 79PCh. 8.8 - A well-insulated shell-and-tube heat exchanger is...Ch. 8.8 - Steam is to be condensed on the shell side of a...Ch. 8.8 - Prob. 82PCh. 8.8 - Prob. 83PCh. 8.8 - Prob. 84PCh. 8.8 - Prob. 85RPCh. 8.8 - Prob. 86RPCh. 8.8 - An aluminum pan has a flat bottom whose diameter...Ch. 8.8 - Prob. 88RPCh. 8.8 - Prob. 89RPCh. 8.8 - A well-insulated, thin-walled, counterflow heat...Ch. 8.8 - Prob. 92RPCh. 8.8 - Prob. 93RPCh. 8.8 - Prob. 94RPCh. 8.8 - Prob. 95RPCh. 8.8 - Nitrogen gas enters a diffuser at 100 kPa and 110C...Ch. 8.8 - Prob. 97RPCh. 8.8 - Steam enters an adiabatic nozzle at 3.5 MPa and...Ch. 8.8 - Prob. 99RPCh. 8.8 - A pistoncylinder device initially contains 8 ft3...Ch. 8.8 - An adiabatic turbine operates with air entering at...Ch. 8.8 - Steam at 7 MPa and 400C enters a two-stage...Ch. 8.8 - Prob. 103RPCh. 8.8 - Steam enters a two-stage adiabatic turbine at 8...Ch. 8.8 - Prob. 105RPCh. 8.8 - Prob. 106RPCh. 8.8 - Prob. 107RPCh. 8.8 - Prob. 108RPCh. 8.8 - Prob. 109RPCh. 8.8 - Prob. 111RPCh. 8.8 - A passive solar house that was losing heat to the...Ch. 8.8 - Prob. 113RPCh. 8.8 - A 4-L pressure cooker has an operating pressure of...Ch. 8.8 - Repeat Prob. 8114 if heat were supplied to the...Ch. 8.8 - Prob. 116RPCh. 8.8 - A rigid 50-L nitrogen cylinder is equipped with a...Ch. 8.8 - Prob. 118RPCh. 8.8 - Prob. 119RPCh. 8.8 - Prob. 120RPCh. 8.8 - Reconsider Prob. 8-120. The air stored in the tank...Ch. 8.8 - Prob. 122RPCh. 8.8 - Prob. 123RPCh. 8.8 - Prob. 124RPCh. 8.8 - Prob. 125RPCh. 8.8 - Prob. 126RPCh. 8.8 - Prob. 127RPCh. 8.8 - Water enters a pump at 100 kPa and 30C at a rate...Ch. 8.8 - Prob. 129RPCh. 8.8 - Prob. 130RPCh. 8.8 - Obtain a relation for the second-law efficiency of...Ch. 8.8 - Writing the first- and second-law relations and...Ch. 8.8 - Prob. 133RPCh. 8.8 - Keeping the limitations imposed by the second law...Ch. 8.8 - Prob. 135FEPCh. 8.8 - Prob. 136FEPCh. 8.8 - Prob. 137FEPCh. 8.8 - Prob. 138FEPCh. 8.8 - A furnace can supply heat steadily at 1300 K at a...Ch. 8.8 - A heat engine receives heat from a source at 1500...Ch. 8.8 - Air is throttled from 50C and 800 kPa to a...Ch. 8.8 - Prob. 142FEPCh. 8.8 - A 12-kg solid whose specific heat is 2.8 kJ/kgC is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A spring package with two springs and an external force, 200N. The short spring has a loin of 35 mm. Constantly looking for spring for short spring so that total compression is 35 mm (d). Known values: Long spring: Short spring:C=3.98 N/mm Lo=65mmLo=87.4mmF=c·fTotal compression is same for both spring. 200 = (3.98(c1) × 35) + (c₂ × 35) 200 = 139.3 + 35c₂ 200 - 139.3 = 35c₂ 60.7 = 35c₂ c₂ = 60.7/35 Short spring (c₂) = 1.73 N/mm According to my study book, the correct answer is 4.82N/mm What is wrong with the calculating?arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- 20. [Ans. 9; 71.8 mm] A semi-elliptical laminated spring is made of 50 mm wide and 3 mm thick plates. The length between the supports is 650 mm and the width of the band is 60 mm. The spring has two full length leaves and five graduated leaves. If the spring carries a central load of 1600 N, find: 1. Maximum stress in full length and graduated leaves for an initial condition of no stress in the leaves. 2. The maximum stress if the initial stress is provided to cause equal stress when loaded. [Ans. 590 MPa ; 390 MPa ; 450 MPa ; 54 mm] 3. The deflection in parts (1) and (2).arrow_forwardQ6/ A helical square section spring is set inside another, the outer spring having a free length of 35 mm greater than the inner spring. The dimensions of each spring are as follows: Mean diameter (mm) Side of square section (mm) Active turns Outer Inner Spring Spring 120 70 8 7 20 15 Determine the (1) Maximum deflection of the two springs and (2) Equivalent spring rate of the two springs after sufficient load has been applied to deflect the outer spring 60 mm. Use G = 83 GN/m².arrow_forwardQ2/ The bumper springs of a railway carriage are to be made of rectangular section wire. The ratio of the longer side of the wire to its shorter side is 1.5, and the ratio of mean diameter of spring to the longer side of wire is nearly equal to 6. Three such springs are required to bring to rest a carriage weighing 25 kN moving with a velocity of 75 m/min with a maximum deflection of 200 mm. Determine the sides of the rectangular section of the wire and the mean diameter of coils when the shorter side is parallel to the axis of the spring. The allowable shear stress is not to exceed 300 MPa and G = 84 kN/mm². Q6/ A belicalarrow_forward
- 11. A load of 2 kN is dropped axially on a close coiled helical spring, from a height of 250 mm. The spring has 20 effective turns, and it is made of 25 mm diameter wire. The spring index is 8. Find the maximum shear stress induced in the spring and the amount of compression produced. The modulus of rigidity for the material of the spring wire is 84 kN/mm². [Ans. 287 MPa; 290 mm]arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- S B Pin 6 mm Garrow_forwardMid-Term Exam 2024/2025 Post graduate/Applied Mechanics- Metallurgy Q1/ State the type of fault in the following case, and state the structure in which it will appear. АВСАВСВАСВАСАВСАВСarrow_forwardالثانية Babakt Momentum equation for Boundary Layer S SS -Txfriction dray Momentum equation for Boundary Layer What laws are important for resolving issues 2 How to draw. 3 What's Point about this.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
What is entropy? - Jeff Phillips; Author: TED-Ed;https://www.youtube.com/watch?v=YM-uykVfq_E;License: Standard youtube license