
Polyvinylpyrrolidone (PVP) is a polymer product used as a binding agent in pharmaceutical applications as well as in personal-care items such as hairspray. In the manufacture of PVP, a spray-drying process is used to collect solid PVP from an aqueous suspension, as shown in the flowchart on the next page. A liquid solution containing 65 wt% PVP and the balance water at 25°C is pumped through an atomizing nozzle at a rate of 1500 kg/h into a stream of preheated air flowing at a rate of 1.57 × 104 SCMH. The water evaporates into the stream of hot air and the solid PVP particles are suspended in the humidified air. Downstream, the particles are separated from the air with a filter and collected. The process is designed so that the exiting solid product and humid air are in thermal
equilibrium with each other at 110°C. For convenience, the spray-drying and solid-separation processes are shown as one unit that may be considered adiabatic.
| Polymer solution25°C | |||
| Humid Air | |||
| 1IO°C | |||
| SPRAY | |||
Air, 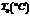 |
DRYER and | Dry solid polymer | |
| SOLID | |||
| SEPARATOR | 110°C | ||
- Draw and completely label the process flow diagram and perform a degree-of-freedom analysis.
Trending nowThis is a popular solution!

Chapter 8 Solutions
ELEM.PRIN.OF CHEM.PROCESS-ACCESS
Additional Engineering Textbook Solutions
Electric Circuits. (11th Edition)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Introduction To Programming Using Visual Basic (11th Edition)
Starting Out with Java: From Control Structures through Data Structures (4th Edition) (What's New in Computer Science)
Starting Out with Python (4th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
- A passive solar house was determined to lose heat to the outdoors at an average rate of 50,000 kJ/h during a typical 10-hour winter night. The house is to be maintained at 22°C at all times. Passive heating is accomplished by 50 glass containers each containing 20 L of water that is heated to 80°C during the day by absorbing solar energy. A 15-kW back-up electric resistance heater turns on whenever necessary to keep the house at 22°C. (a) How many hours does the electric heating system run during a typical winter night? (b) How many hours would the electric heater run during a typical winter night if the house did not have passive solar heating? For the density and specific heat of water at room temperature, use p = 1 kg/L and cp = 4.18 kJ/kg.°Carrow_forwardA well-insulated rigid tank contains 3 kg of a saturated liquid-vapor mixture of water at 200 kPa. Initially, three-quarters of the mass is in the liquid phase. An electric resistance heater placed in the tank is now turned on and kept on until all the liquid in the tank is vaporized. Determine the entropy change of the water during this process.arrow_forwardHeat in the amount of 100 kJ is transferred directly from a hot reservoir (heat source) at 1200 K to a cold reservoir (heat sink) at 600 K. Calculate the entropy change of the two reservoirs and determine if the second law of thermodynamics is satisfied.arrow_forward
- The following chemical reaction takes place at 500K and 1 atm and the products leaves at 1000K aCH4 + b(O2 + 3.76N2)=7.7CO2 + 0.5CO + 2CH4+2.95O2 + 86.85N2 + cH2O use the specific heat capacity given in Table A-21 (Moran and Shapiro, page 755) and the heat of formation given in Tabble A-25 (Moran and Shapiro, page 763) determine: 1. The stoichiometric coefficients (a, b, and c) 2. The air-fuel ratio on a molar basis 3. The air-fuel ratio on a mass basis 4. The stoichiometric air fuel ratio 5. The excess air (%) 6. The lower heating value 7. The rate of heat transfer from the combustion chamber.arrow_forward3. Nitric oxide is produced in the body by several different enzymes and acts as a signal that controls blood pressure, long-term memory, and other critical functions. The major route for removing NO from biological fluids is via reaction with O2 to give NO₂ 2NO(g) + O2(g) → 2NO2(g) The following table lists kinetics data for the reaction of NO with O2 at 25°C: Experiment 1 [NO] (M) 0.0235 2 0.0235 3 0.0470 4 0.0470 (a) Determine the rate law for the reaction (b) calculate the rate constant. [02]0 (M) Initial Rate (M/s) 0.0125 7.98 × 10-3 0.0250 15.9 × 10-3 0.0125 32.0 × 10-3 0.0250 63.5 x 10-3 5:32arrow_forwardA closed system of 122 moles of an ideal gas with constant-pressure heat capacity of cp = 2.5R expands isobarically from 52°C and 4.9 bar to 137°C, with a thermodynamic efficiency of 0.74. How much total work is involved in this process? Please report your answer to the nearest whole kJ and don't forget the sign: "-" if the work is negative, no sign if the work is positive.arrow_forward
- Liquid toluene at 20°C is reversibly and isothermally compressed from 2.94 bar to 7.7 bar. What is the specific work, in J/kg, required to accomplish this? Some properties of liquid toluene at 20°C: β = 1.05 x 10-3 ºC-1 , κ = 8.96 x 10-5 bar-1 , V = 1154 cm3 kg-1. Please report your answer to 3 SF. Be very, very careful of units!arrow_forward132 kJ of work is transferred from a system to its surroundings in a reversible process to get it from state A to state B. If a similar but irreversible process is performed from state A to state B with a thermodynamic efficiency of 0.73, how much work will be transferred, in kJ? Be sure to include the correct sign on your answer: if it is positive, do NOT include a "+", but if it is negative you MUST include a "−" sign.arrow_forward2- What will be the power required to crush 150 tonnes per hour of limestone if 80 percent of the feed passes 50 mm screen and 80 percent of the product a 3.125 mm screen? Work index of limestone 12.74.arrow_forward
- 3- A certain crusher accepts a feed material having a volume-surface mean diameter of 19 mm and gives a product of volume-surface mean diameter of 5 mm. The power required to crush 15 tonnes per hour is 7.5 kW. What will be the power consumption if the capacity is reduced to 12 tonnes per hour?arrow_forwardCR = CAOK1 K2-K1 - Cs CAO CR - CA = [e-k₁t + e-k₂t] --(6) Cs = Cao CAO 1+ K₂e-kit K₁e-k2t + K1-K2 K₂-K1 By differentiating eq (6) and set to zero (dCR = 0), the time at which concentration of R occurs is thus: dT K2 1 In Ki K1 tmax K₂-K1 Klogmean (7) Equation 7. Prove that?arrow_forwardQuestion #6 a) Draw a simple block flow diagram of a petroleum refinery consisting of following sections. 1. Atmospheric and vacuum distillation 2. Hydrotreating of diesel steam 3. Hydrocracking of LVGO Show main product streams from each unit. (8)arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





