
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.12P
What
a.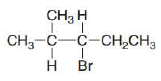 b.
b.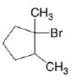 c.
c.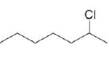 d.
d.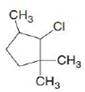
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Some of the theories used to describe interface structure can be distinguished by:1. the measured potential difference.2. the distribution of ions in solution.3. the calculation of charge density.4. the external Helmoltz plane.
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Chapter 8 Solutions
Organic Chemistry
Ch. 8 - Label the and carbons in each alkyl halide. Draw...Ch. 8 - Problem 8.2 Classify each alkene in the following...Ch. 8 - Prob. 8.3PCh. 8 - Prob. 8.4PCh. 8 - Problem 8.5 Label each pair of alkenes as...Ch. 8 - Prob. 8.6PCh. 8 - Problem 8.7 Several factors can affect alkene...Ch. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10P
Ch. 8 - Prob. 8.11PCh. 8 - What alkenes are formed from each alkyl halide by...Ch. 8 - Prob. 8.13PCh. 8 - Prob. 8.14PCh. 8 - Problem 8.15 How does each of the following...Ch. 8 - Prob. 8.16PCh. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Problem 8.19 Explain why...Ch. 8 - Prob. 8.21PCh. 8 - Draw the alkynes formed when each dihalide is...Ch. 8 - Prob. 8.23PCh. 8 - Draw a stepwise mechanism for the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 8.26PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 8.28PCh. 8 - Prob. 8.29PCh. 8 - Prob. 8.30PCh. 8 - Prob. 8.31PCh. 8 - PGF2 is a prostaglandin, a group of compounds that...Ch. 8 - Prob. 8.33PCh. 8 - Prob. 8.34PCh. 8 - Prob. 8.35PCh. 8 - Prob. 8.36PCh. 8 - Prob. 8.37PCh. 8 - Prob. 8.38PCh. 8 - Prob. 8.39PCh. 8 - Prob. 8.40PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 8.42PCh. 8 - Prob. 8.43PCh. 8 - Prob. 8.44PCh. 8 - 8.42 In the dehydrohalogenation of...Ch. 8 - Prob. 8.46PCh. 8 - Prob. 8.47PCh. 8 - Prob. 8.48PCh. 8 - Prob. 8.49PCh. 8 - Prob. 8.50PCh. 8 - Draw the products of each reaction. a.c. b.d.Ch. 8 - Draw the structure of a dihalide that could be...Ch. 8 - Under certain reaction conditions, 2,...Ch. 8 - For which reaction mechanisms, SN1, SN2, E1 or...Ch. 8 - Draw the organic products formed in each reaction....Ch. 8 - Prob. 8.56PCh. 8 - Prob. 8.57PCh. 8 - Draw all products, including stereoisomers, in...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 8.60PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 8.66PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 8.68PCh. 8 - Although dehydrohalogenation occurs with anti...Ch. 8 - 8.68 (a) Draw all products formed by treatment of...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forwardIn the two chair conformations of glucose, the most stable is the one with all the OH groups in the equatorial position. Is this correct?arrow_forwardIndicate the formula of the product obtained by reacting D-Galactose with hydroxylamine.arrow_forward
- helparrow_forwardThe temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY