
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of
salts with organic compounds, the most familiar is a two-stage procedure for alkene hydration called oxymercuration–demercuration. Its application in the conversion of
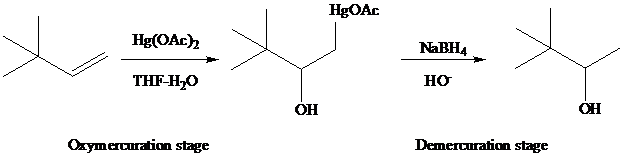
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with
of the alkene. The oxygen of water, one of the components in the
solvent mixture, bonds to
to
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of
and
characterizes the oxymercuration stage, and
4. the replacement of
c by H in the demercuration stage is not stereospecific.
The structure of the intermediate in oxymercuration has received much attention and can be
approached by considering what is likely to happen when the electrophile
reacts with the double bond of an alkene.
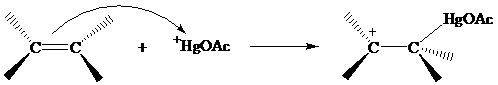
Recall from Section
that electrons in bonds that are
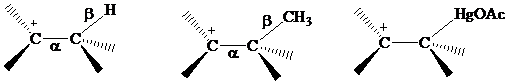
The electrons in a
or
electrons, making
stabilization by hyperconjugation more effective for
or
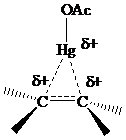
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.
Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than
Oxymercuration–demercuration of allyl alcohol gives
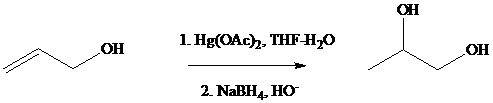
Under the same conditions, however,
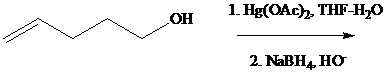
What is the most reasonable structure for the product of this reaction?
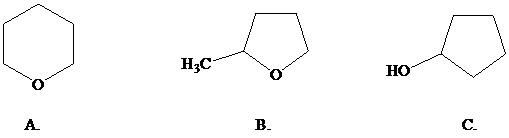
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Provide the IUPAC name of the following molecule. Don't forget to include the proper stereochemistry where appropriate.arrow_forward3. 2. 1. On the graph below, plot the volume of rain in milliliters versus its height in centimeters for the 400 mL beaker. Draw a straight line through the points and label it "400 mL beaker." Volume (mL) 400 350 300 250 200 150 750 mL Florence Volume Versus Height of Water 400 mL beaker 100 50 0 0 2 3 4 5 Height (cm) 6 7 8 9 10 Explain why the data points for the beaker lie roughly on a straight line. What kind of relationship is this? How do you know? (see page 276 text) the design of the beaker is a uniform cylinder the volume of liquid increases evenly with its height resulting in a linear relationship. What volume would you predict for 10.0 cm of water? Explain how you arrived at your answer. Use the data table and the graph to assist you in answering the question. 4. Plot the volume of rain in milliliters versus its height in centimeters for the 250 mL Florence flask on the same graph. Draw a best-fit curve through the points and label it "250 mL Florence flask." oke camearrow_forwardShow work. Don't give Ai generated solutionarrow_forward
- In the video, we looked at the absorbance of a certain substance and how it varies depending on what wavelength of light we are looking at. Below is a similar scan of a different substance. What color BEST describes how this substance will appear? Absorbance (AU) Violet Blue Green Orange 1.2 1.0- 0.8- 0.6- 0.4- 0.2 0.0 450 500 550 600 650 700 Wavelength (nm) violet indigo blue green yellow orange red Red O Cannot tell from this information In the above graph, what causes -450 nm wavelength of light to have a higher absorbance than light with a -550 nm wavelength? Check all that are true. The distance the light travels is different The different data points are for different substances The concentration is different at different times in the experiment Epsilon (molar absortivity) is different at different wavelengthsarrow_forward5. a. Data were collected for Trial 1 to determine the molar mass of a nonvolatile solid solute when dissolved in cyclo- hexane. Complete the table for the analysis (See Report Sheet). Record calculated values with the correct number of significant figures. B. Freezing Point of Cyclohexane plus Calculation Zone Unknown Solute 2. Mass of cyclohexane (g) 10.14 Part C.4 3. Mass of added solute (g) 0.255 C. Calculations 1. k; for cyclohexane (°C⚫ kg/mol) 20.0 2. Freezing point change, AT, (°C) 3.04 Part C.6 3. Mass of cyclohexane in solution (kg) 4. Moles of solute, total (mol) Show calculation. 5. Mass of solute in solution, total (g) 6. Molar mass of solute (g/mol) Show calculation.arrow_forwardDraw and name the R groups of all 20 amino acids.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


