
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of Hg(II) salts with organic compounds, the most familiar is a two-stage procedure for alkene hydration called oxymercuration–demercuration. Its application in the conversion of 3,3-dimethyl-1-butene to 3,3-dimethy-2-butanol illustrates the procedure.
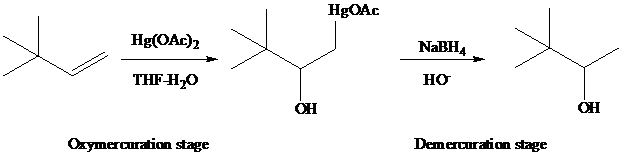
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with mercury(II) acetate [Hg(O2CCH3)2, abbreviated as Hg(OAc)2]. mercury(II) acetate is a source of the electrophile H+gOAc, which bonds to C-1 of the alkene. The oxygen of water, one of the components in the THF–H2O solvent mixture, bonds to C-2. The demercuration operation uses sodium borohydride (NaBH4, a reducing agent) to convert C - Hg to C - H.
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of HgOAc and OH characterizes the oxymercuration stage, and
4. the replacement of HgOAc c by H in the demercuration stage is not stereospecific.
The structure of the intermediate in oxymercuration has received much attention and can beapproached by considering what is likely to happen when the electrophile H+gOAc reacts with the double bond of an alkene.
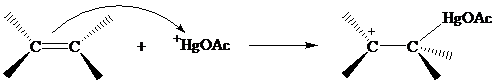
Recall from Section 5.9 that electrons in bonds that are β to a positively charged carbon stabilize a carbocation by hyperconjugation.
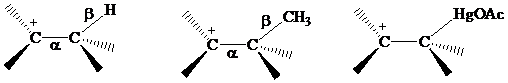
The electrons in a C - Hg σs bond are more loosely held than C - H or C - C electrons, makingstabilization by hyperconjugation more effective for β-C-Hg than for β-C-H or β-C - C. Hyperconjugative stabilization of the intermediate in oxymercuration is normally shown using dashed lines to represent partial bonds. The intermediate is referred to as a “bridged” mercurinium ion.
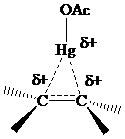
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than Hg(OAc)2, the nucleophile may be other than H2O, and the reaction may be intramolecular rather than intermolecular.
Oxymercuration–demercuration of 1-methylcyclopentene gives which of the following products?
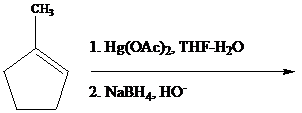
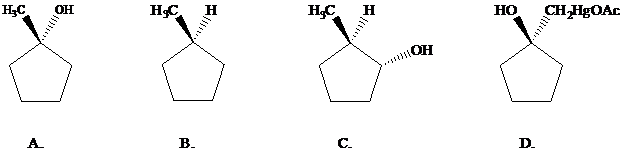
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


