
Concept explainers
Draw Lewis structures for the following molecules and ions:
Interpretation:
The Lewis structures of the given molecules and ions are to be drawn.
Concept introduction:
In Lewis dot symbol, for each element, dots are mentioned around the symbol of an atom.
In Lewis dot symbol, valence electrons are represented by dots.
Dots are placed above and below as well as to the left and right of symbol.
Number of dots is important in Lewis dot symbol but not the order in which the dots are placed around the symbol.
In writing symbol pairing is not done until absolutely necessary.
For metals, the number of dots represents the number of electrons that are lost when the atom forms a cation.
For second period nonmetals, the number of unpaired dots is the number of bonds the atom can form.
Atomic ions can also be represented by dot symbols, by simply adding (for anions) and subtracting (for cations) the appropriate number of dots from Lewis dot symbol.
Lewis structure is the representation of bonding and nonbonding electron pairs present in the outermost shell of all atoms present in the molecule.
The number of bonds formed by an atom in the molecule is determined by the valence electron pairs.
Answer to Problem 43QP
Solution:
a)
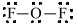
b)
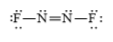
c)
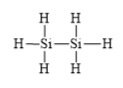
d)
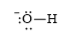
e)
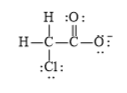
f)
Explanation of Solution
a)
The electronic configuration of oxygen and fluorine in
The oxygen atom contains fourvalence electrons in its
The Lewis structure of
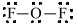
b)
The electronic configuration of nitrogen and fluorine in
The nitrogen atom contains three valence electrons in its
The Lewis structure of
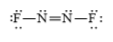
c)
The electronic configuration of silicon and hydrogen in
The silicon atom has a tendency to form four bonds because of the presence of four valence electrons in its outermost shell and hydrogen has a tendency to form one bond because of the presence of one electron in its outermost shell.
The Lewis structure is as follows:
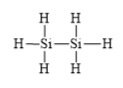
d)
The electronic configuration of oxygen and hydrogen in
The oxygen atom contains four valence electrons in its
The Lewis structure of
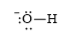
e)
The electronic configuration of oxygen, carbon, chlorine, and hydrogen in
The carbon atom has a tendency to form four bonds because of the presence of four electrons in its outermost shell, hydrogen has a tendency to form one bond because of the presence of one electron in its outermost shell, chlorine has atendency to form one bond because of the presence of five electrons in its
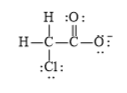
f)
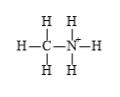
The electronic configuration of carbon, nitrogen, and hydrogen in
The carbon atom has a tendency to form four bonds because of the presence of four electrons in its outermost shell, hydrogen has a tendency to form one bond because of the presence of one electron in its outermost shell, and nitrogen has a tendency to form four bonds due to the presence of three electrons in its

Want to see more full solutions like this?
Chapter 8 Solutions
CHEMISTRY >CUSTOM<
- Draw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forwardDraw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forward
- Draw the monomers required to synthesize this condensation polymer please.arrow_forwardProvide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forward
- Identify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forwardH- H H H H H H Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H H H A. H H H H-C CI H H D. H H H H H H C C -H H C C H H H H B. H CI H H- C C H H H H E. H CI H C.arrow_forwardWhy doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?arrow_forward
- 6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform has absorption bands at: 350 nm (absorbance A = 2.34); 514 nm(absorbance A = 0.0532); Calculate the molar absorptivity values for these bands. Comment on their possible nature (charge transfer transitions or d-d S N- transitions?). (4 points)arrow_forwardWhat is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

