
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
14th Edition
ISBN: 9780136873891
Author: Brown
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 3E
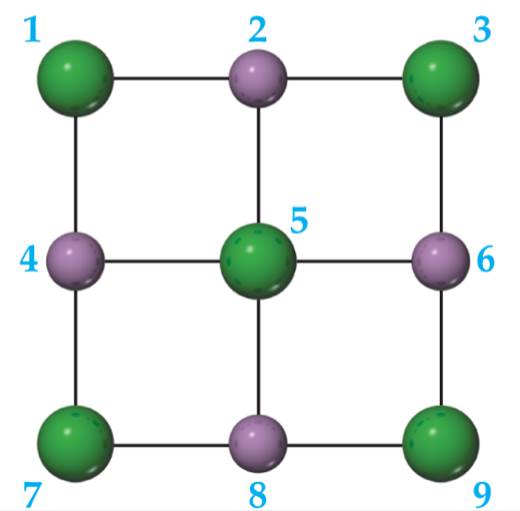
A portion of a two-dimensional "slab" of NaCl(s) is shown here (see Figure 8.2) in which the ions are numbered.
- Which colored balls must represent sodium ions?
- Which colored balls must represent chloride ions?
- Consider ion 5. How many attractive electrostatic interactions are shown for it?
- Consider ion 5. How many repulsive interactions are shown for it?
- Is the sum of the attractive interactions in part (c) larger or smaller than the sum of the repulsive interactions in part (d)?
- If this pattern of ions was extended indefinitely in two dimensions, would the lattice energy be positive or negative? [Section 8.2]
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2. Draw mechanisms for the following reactions.
mg
Et
CO₂Hot
H30t
Et
0
Please correct answer and don't use hand rating
Convert the following structures into a chair representation. Then conduct a chair flip.
Cl
a.
b.
C\....
о
Chapter 8 Solutions
CHEMISTRY THE CENTRAL SCIENCE >EBOOK<
Ch. 8.2 - Which of the these elements is most likely to from...Ch. 8.2 - Prob. 8.1.2PECh. 8.2 - Which of the following bond is the most polar? H-F...Ch. 8.2 - Prob. 8.2.2PECh. 8.3 - Prob. 8.3.1PECh. 8.3 - Prob. 8.3.2PECh. 8.4 - Which of the following bonds is the most polar? a....Ch. 8.4 - Which of the following bonds is most polar: S-Cl,...Ch. 8.4 - Prob. 8.5.1PECh. 8.4 - The dipole moment of chlorine monofluoride,...
Ch. 8.5 - Which of the these molecules has a Lewis structure...Ch. 8.5 -
How many valence electrons should appear in the...Ch. 8.5 - Compare the lewis symbol for neon the structure...Ch. 8.5 - Prob. 8.7.2PECh. 8.5 - Prob. 8.8.1PECh. 8.5 - Prob. 8.8.2PECh. 8.5 - Prob. 8.9.1PECh. 8.5 - Prob. 8.9.2PECh. 8.6 - Which of the statements about resonance is true?...Ch. 8.6 - Prob. 8.10.2PECh. 8.7 - Prob. 8.11.1PECh. 8.7 - Prob. 8.11.2PECh. 8 - Prob. 1DECh. 8 - Prob. 1ECh. 8 - Prob. 2ECh. 8 - A portion of a two-dimensional "slab" of NaCl(s)...Ch. 8 - Prob. 4ECh. 8 - Prob. 5ECh. 8 - Incomplete Lewis structures for the nitrous acid...Ch. 8 - Prob. 7ECh. 8 - Prob. 8ECh. 8 - Prob. 9ECh. 8 - True or false: The hydrogen atom is most stable...Ch. 8 - Consider the element silicon, Si. Write its...Ch. 8 - Write the electron configuration for the element...Ch. 8 - Prob. 13ECh. 8 - What is the Lewis symbol for each of the following...Ch. 8 - Using Lewis symbols, diagram the reaction between...Ch. 8 - Use Lewis symbols to represent the reaction that...Ch. 8 - Predict the chemical formula of the ionic compound...Ch. 8 - Prob. 18ECh. 8 - Prob. 19ECh. 8 - Prob. 20ECh. 8 - Is lattice energy usually endothermic or...Ch. 8 - NaCI and KF have the same crystal structure. The...Ch. 8 - Prob. 23ECh. 8 - Prob. 24ECh. 8 - Consider the ionic compounds KF, NaCl, NaBr, and...Ch. 8 - Which of the following trends in lattice energy is...Ch. 8 - Energy is required to remove two electrons from Ca...Ch. 8 - Prob. 28ECh. 8 - Use data from Appendix C, Figure 7.10, and Figure...Ch. 8 - Prob. 30ECh. 8 - Prob. 31ECh. 8 - Prob. 32ECh. 8 - Using Lewis symbols and Lewis structures, diagram...Ch. 8 - Use Lewis symbols and Lewis structures to diagram...Ch. 8 - Prob. 35ECh. 8 - Prob. 36ECh. 8 - Prob. 37ECh. 8 - What is the trend in electronegativity going from...Ch. 8 - Prob. 39ECh. 8 - By referring only to the periodic table, select...Ch. 8 - which of the following bonds are polar? B-F,...Ch. 8 - Arrange the bonds in each of the following sets in...Ch. 8 - Prob. 43ECh. 8 - Prob. 44ECh. 8 - In the following pairs of binary compounds,...Ch. 8 - Prob. 46ECh. 8 - Prob. 47ECh. 8 - Write Lewis structures for the following: H2CO...Ch. 8 - Prob. 49ECh. 8 - Draw the dominant Lewis structure for the...Ch. 8 - Write Lewis structures that obey the octet rule...Ch. 8 - Prob. 52ECh. 8 - Prob. 53ECh. 8 - Prob. 54ECh. 8 - Prob. 55ECh. 8 - Prob. 56ECh. 8 - Prob. 57ECh. 8 - Prob. 58ECh. 8 - Prob. 59ECh. 8 - Prob. 60ECh. 8 - Prob. 61ECh. 8 - 8.62 For Group 3A-7A elements in the third row of...Ch. 8 - Draw the Lewis structures for each of the...Ch. 8 - Prob. 64ECh. 8 - In the vapor phase, BeCl2exists as a discrete...Ch. 8 -
8.66
Describe the molecule xenon trioxide, XeO3,...Ch. 8 -
8.67 There are many Lewis structures you could...Ch. 8 - Prob. 68ECh. 8 - Using Table 8.3, estimate H for each of the...Ch. 8 - Using Table 8.3, estimate H for the following...Ch. 8 - State whether each of these statements is true or...Ch. 8 - Prob. 72ECh. 8 - Prob. 73ECh. 8 - Prob. 74ECh. 8 - Prob. 75ECh. 8 - Prob. 76ECh. 8 - A new compound is made that has a C-C bond length...Ch. 8 - A new compound is made that has an N-N bond length...Ch. 8 - Prob. 79AECh. 8 - Prob. 80AECh. 8 - An ionic substance of formula MX has a lattice...Ch. 8 - Prob. 82AECh. 8 - Prob. 83AECh. 8 - Prob. 84AECh. 8 - Consider the collection of nonmetallic elements 0,...Ch. 8 - The substance chlorine monoxide, CIO(g), is...Ch. 8 -
[8.87]
a. using the electronegativities of Br...Ch. 8 - Prob. 88AECh. 8 - Although I3- is a known ion, F3- is not. a. Draw...Ch. 8 - Calculate the formal charge on the indicated atom...Ch. 8 - The hypochlorite ion, CIO- , is the active...Ch. 8 - Prob. 92AECh. 8 - a. Triazine, C3 H3N3, is like benzene except that...Ch. 8 - Prob. 94IECh. 8 - Prob. 95IECh. 8 - Prob. 96IECh. 8 - Prob. 97IECh. 8 - Prob. 98IECh. 8 - Prob. 99IECh. 8 - Prob. 100IECh. 8 - Prob. 101IECh. 8 - Prob. 102IECh. 8 -
8.103 The compound chloral hydrate, known in...Ch. 8 - Barium azide is 62.04% Ba and 37.96% N. Each azide...Ch. 8 - Acetylene (C2H2) and nitrogen (N2) both contain a...Ch. 8 - Prob. 106IECh. 8 - Prob. 107IECh. 8 -
8.108 Formic acid has the chemical formula...Ch. 8 - Prob. 109IECh. 8 - Prob. 110IE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aktiv Learning App Cengage Digital Learning Part of Speech Table for Assign x o Mail-Karen Ento-Outlook * + app.aktiv.com Your Aktiv Learning trial expires on 02/06/25 at 01:15 PM Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Problem 17 of 30 Drawing Arrows heat 4 O M B D 5x H H Und Settings H Done :0: H Jararrow_forwardConvert the following chairs into ring representations: a. Brz b.arrow_forwardDrawing Arrows 1 I I 1 heat 1 51 MO + Drag To Und Settings Done 0 0 Jan 31 3:5arrow_forward
- Don't used hand raitingarrow_forwardGramicidin A can adopt more than one structure; NMR spectroscopy has revealed an “end-to-end” dimer form, and x-ray crystallography has revealed an “anti-parallel double- helical” form. Briefly outline and describe an experimentalapproach/strategy to investigate WHICH configuration (“end-to-end dimer” vs “anti-paralleldouble helical”) gramicidin adopts in an actual lipid bilayer.arrow_forwardDon't used hand raitingarrow_forward
- CHEM2323 Problem 2-24 Tt O e: ל Predict the product(s) of the following acid/base reactions. Draw curved arrows to show the formation and breaking of bonds. If the bonds needed are not drawn out, you should redraw them. + BF3 (a) (b) HI + (c) OH -BF Problem 2-25 Use curved arrows and a proton (H+) to draw the protonated form of the following Lewis bases. Before starting, add all missing lone pairs. (a) (b) :0: (c) N 1 CHEM2323 PS CH02 Name:arrow_forwardCHEM2323 Problem 2-26 Tt O PS CH02 Name: Use the curved-arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right. (Draw all lone pairs first) (a) NH2 NH2 + (b) Problem 2-27 Double bonds can also act like Lewis bases, sharing their electrons with Lewis acids. Use curved arrows to show how each of the following double bonds will react with H-Cl and draw the resulting carbocation. (a) H2C=CH2 (b) (c) Problem 2-28 Identify the most electronegative element in each of the following molecules: (a) CH2FCI F Problem 2-29 (b) FCH2CH2CH2Br (c) HOCH2CH2NH2 (d) CH3OCH2Li F 0 0 Use the electronegativity table in Figure 2.3 to predict which bond in the following pairs is more polar and indicate the direction of bond polarity for each compound. (a) H3C-Cl or Cl-CI (b) H3C-H or H-CI (c) HO-CH3 or (CH3)3Si-CH3 (d) H3C-Li or Li-OHarrow_forwardDon't used hand raitingarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Types of bonds; Author: Edspira;https://www.youtube.com/watch?v=Jj0V01Arebk;License: Standard YouTube License, CC-BY