
Interpretation: The principle behind the working of barometer and manometer has to be explained.
Concept Introduction:
The force acting per unit area is called as Pressure. Differential pressure is indicated by measuring devices i.e. in relative with the atmosphere pressure. This is known as gauge pressure. The calculated pressure can either be positive or negative with respect to the atmospheric pressure. Vacuum is generally known as a negative gauge pressure.
Atmospheric pressure is measured by using an instruments called as barometer and manometer.
Explanation of Solution
Explanation
A barometer consists of Mercury column that is tipped inverted and positioned in a dish containing Mercury. The Mercury in the column moves to and fro. The measure of atmospheric pressure is done by the height of the column. There is a downward force produced by the weight of the Mercury, this downward force pushes the mercury to fall out of the column. But, there is repulsive force that keeps Mercury in the column. This repulsive force is due to the atmospheric gas particles that collide with the surface of the Mercury in the dish; this makes the mercury to get pushed up in the column. The level of Mercury in the column stays constant, when the two repulsive forces are same in strength to each other. The Mercury’s constant height is supported by the atmosphere is a measure of pressure of the atmosphere.
Manometer: A manometer also has two repulsive forces against each other. A force is exerted by the gas molecules in the flask. The repulsive force is seen on the further region of the Mercury filled U tube; that is force exerted by atmospheric gases. The measure of the variation in pressure between the gas in the flask and the atmosphere gives the difference in height of the Mercury in the U tube. The greater or lesser the gas in the flask to the atmosphere pressure can be determined by measuring the difference in the height of the Mercury.
Explain the working of Barometer.
A barometer consists of Mercury column that is tipped inverted and positioned in a dish containing Mercury. The Mercury in the column moves to and fro. The measure of atmospheric pressure is done by the height of the column. There is a downward force produced by the weight of the Mercury, this downward force pushes the mercury to fall out of the column. But, there is repulsive force that keeps Mercury in the column. This repulsive force is due to the atmospheric gas particles that collide with the surface of the Mercury in the dish; this makes the mercury to get pushed up in the column. The level of Mercury in the column stays constant, when the two repulsive forces are same in strength to each other. The Mercury’s constant height is supported by the atmosphere is a measure of pressure of the atmosphere.
A simple barometer is illustrated in the figure 1,
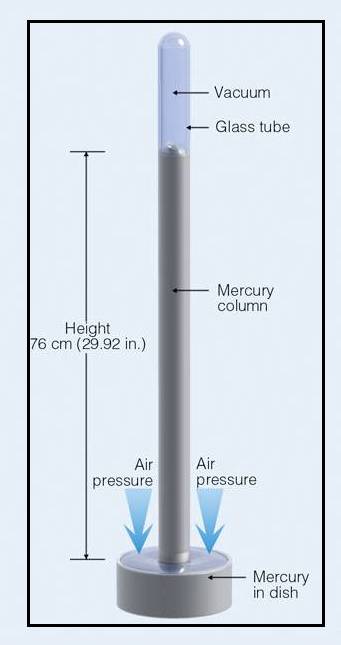
Figure 1: Simple Barometer
Explain the working of Manometer
A manometer also has two repulsive forces against each other. A force is exerted by the gas molecules in the flask. The repulsive force is seen on the further region of the Mercury filled U tube; that is force exerted by atmospheric gases. The measure of the variation in pressure between the gas in the flask and the atmosphere gives the difference in height of the Mercury in the U tube. The greater or lesser the gas in the flask to the atmosphere pressure can be determined by measuring the difference in the height of the Mercury.
Illustration of simple manometer is shown in figure 2,
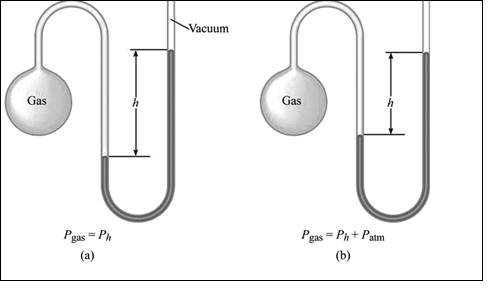
Figure 2: A simple manometer
Conclusion
The principle and working of barometer and manometer are explained.
Want to see more full solutions like this?
Chapter 8 Solutions
EBK CHEMISTRY: AN ATOMS FIRST APPROACH
- 1. Part 1: Naming Organic Compounds он H₁C-C-CH3 CH3 Br CI CI 2. Br-CH-CH-CH₂ H₂C-CH-C= -CH-CH2-CH3 3. HC-CH-CH-C-OH 5. H₂C-CH-CH₂-OH 7. OH 4. CH CH₂-CH₂ 6. сно CH-CH-CH-CH₂-CH₂ H₁₂C-CH-CH-CH-CH₁₂-CH₁₂ 8. OHarrow_forward11 Organic Chemistry Organic Nomenclature Practice Name/Functional Group n-butane Formula Structural Formula (1) C4tt10 H3C C- (2) CH3CH2CH2 CH 3 H₂ -CH3 Н2 name & functional group (1) and (2) OH H₁₂C Н2 name only (1) and (2) name only (1) and (2) H₁C - = - CH₂ Н2 HC=C-C CH3arrow_forwardUnder aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forward
- Assign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forwardPredict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forward
- Predict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forwardWhat is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward
- 个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forwardPredict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





