
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.54SP
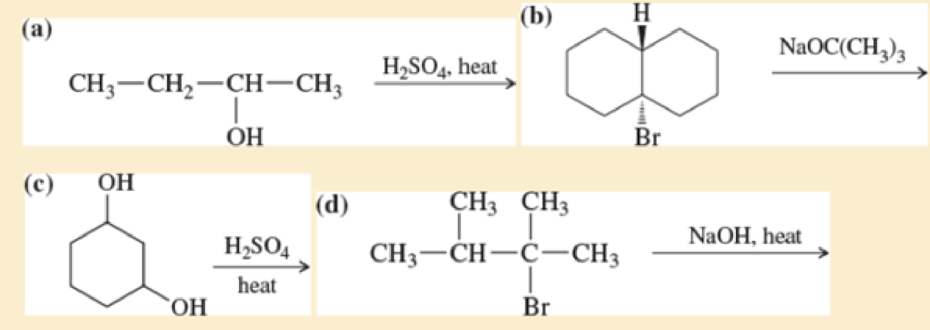
Write a balanced equation for each reaction, showing the major product you expect.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the
pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes.
pressure (atm)
2
0
0
200
400
temperature (K)
X
QUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes
*The values are all provided in the photo attached*
pressure (atm)
3
The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the
temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes.
0
0
200
temperature (K)
400
а
Chapter 7 Solutions
Organic Chemistry (9th Edition)
Ch. 7.3A - Prob. 7.1PCh. 7.3A - Prob. 7.2PCh. 7.3B - Draw five more compounds of formula C4H6NOC1.Ch. 7.3B - For each of the following molecular formulas,...Ch. 7.4 - Give the systematic (IUPAC) names of the following...Ch. 7.5B - The following names are all incorrect. Draw the...Ch. 7.5B - Prob. 7.8PCh. 7.5B - a. How many stereogcmc double bonds are in...Ch. 7.6 - Teflon-coated frying pans routinely endure...Ch. 7.7B - Prob. 7.11P
Ch. 7.8B - Use the data in Table7-2 to predict the energy...Ch. 7.8C - Prob. 7.13PCh. 7.8E - Explain why each of the following alkenes is...Ch. 7.8F - Prob. 7.15PCh. 7.10 - Prob. 7.16PCh. 7.10A - SN1 substitution and E1 elimination frequently...Ch. 7.10C - Prob. 7.18PCh. 7.10C - Prob. 7.19PCh. 7.10C - Prob. 7.20PCh. 7.11 - Prob. 7.21PCh. 7.11 - Prob. 7.22PCh. 7.12 - Prob. 7.23PCh. 7.12 - Prob. 7.24PCh. 7.13 - Prob. 7.25PCh. 7.14B - Prob. 7.26PCh. 7.14B - Make models of the blowing compounds, and predict...Ch. 7.15 - Prob. 7.28PCh. 7.15 - Prob. 7.29PCh. 7.15 - Prob. 7.30PCh. 7.15 - Prob. 7.31PCh. 7.16 - Predict the major and minor elimination products...Ch. 7.17B - Predict the products and mechanisms of the...Ch. 7.18 - Propose mechanisms for the following reactions.Ch. 7.18 - Prob. 7.35PCh. 7.19B - The dehydrogenation of butane to trans-but-2-ene...Ch. 7.19B - Prob. 7.37PCh. 7.19B - Prob. 7.38PCh. 7.19B - Prob. 7.39PCh. 7 - Prob. 7.40SPCh. 7 - Prob. 7.41SPCh. 7 - Prob. 7.42SPCh. 7 - Prob. 7.43SPCh. 7 - Prob. 7.44SPCh. 7 - Prob. 7.45SPCh. 7 - Prob. 7.46SPCh. 7 - The energy difference between cis- and...Ch. 7 - Prob. 7.48SPCh. 7 - Prob. 7.49SPCh. 7 - Prob. 7.50SPCh. 7 - What halides would undergo E2 dehydrohalogenation...Ch. 7 - Prob. 7.52SPCh. 7 - Prob. 7.53SPCh. 7 - Write a balanced equation for each reaction,...Ch. 7 - Prob. 7.55SPCh. 7 - Using cyclohexane as your starting material, show...Ch. 7 - Show how you would prepare cyclopentene from each...Ch. 7 - Prob. 7.58SPCh. 7 - E1 eliminations of alkyl halides are rarely useful...Ch. 7 - Prob. 7.60SPCh. 7 - Propose mechanisms for the following reactions....Ch. 7 - Prob. 7.62SPCh. 7 - Prob. 7.63SPCh. 7 - Prob. 7.64SPCh. 7 - Prob. 7.65SPCh. 7 - Prob. 7.66SPCh. 7 - Prob. 7.67SPCh. 7 - Prob. 7.68SPCh. 7 - Prob. 7.69SPCh. 7 - Explain the dramatic difference in rotational...Ch. 7 - One of the following dichloronorbornanes undergoes...Ch. 7 - A graduate student wanted to make...Ch. 7 - Prob. 7.73SPCh. 7 - Prob. 7.74SPCh. 7 - Prob. 7.75SPCh. 7 - Prob. 7.76SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Match the people in column A to their contribution toward the advancement of microbiology, in column B. Column ...
Microbiology: An Introduction
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Some people compare DNA to a blueprint stored in the office of a construction company. Explain how this analogy...
Biology: Concepts and Investigations
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- er your payment details | bar xb Home | bartleby x + aleksogi/x/isl.exe/1o u-lgNskr7j8P3jH-1Qs_pBanHhviTCeeBZbufuBYT0Hz7m7D3ZcW81NC1d8Kzb4srFik1OUFhKMUXzhGpw7k1 O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 1 3- 0- 0 200 Explanation Check temperature (K) 400 X Q Search L G 2025 McGraw Hill LLC. All Rights Reserved Terms of Use Privacy Cearrow_forward5.arrow_forward6.arrow_forward
- 0/5 alekscgi/x/sl.exe/1o_u-IgNglkr7j8P3jH-IQs_pBaHhvlTCeeBZbufuBYTi0Hz7m7D3ZcSLEFovsXaorzoFtUs | AbtAURtkqzol 1HRAS286, O States of Matter Sketching a described thermodynamic change on a phase diagram The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 3 pressure (atm) + 0- 0 5+ 200 temperature (K) 400 Explanation Check X 0+ F3 F4 F5 F6 F7 S 2025 McGraw Hill LLC All Rights Reserved. Terms of Use Privacy Center Accessibility Q Search LUCR + F8 F9 F10 F11 F12 * % & ( 5 6 7 8 9 Y'S Dele Insert PrtSc + Backsarrow_forward5.arrow_forward9arrow_forward
- alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZS18w-nDB10538ZsAtmorZoFusYj2Xu9b78gZo- O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 3- 200 temperature (K) Explanation Chick Q Sowncharrow_forward0+ aleksog/x/lsl.exe/1ou-lgNgkr7j8P3H-IQs pBaHhviTCeeBZbufuBYTOHz7m7D3ZStEPTBSB3u9bsp3Da pl19qomOXLhvWbH9wmXW5zm O States of Matter Sketching a described thermodynamic change on a phase diagram 0/5 Gab The temperature on a sample of pure X held at 0.75 atm and -229. °C is increased until the sample sublimes. The temperature is then held constant and the pressure is decreased by 0.50 atm. On the phase diagram below draw a path that shows this set of changes. F3 pressure (atm) 0- 0 200 Explanation temperature (K) Check F4 F5 ☀+ Q Search Chill Will an 9 ENG F6 F7 F8 F9 8 Delete F10 F11 F12 Insert PrtSc 114 d Ararrow_forwardx + LEKS: Using a phase diagram a X n/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3jH-IQs_pBan HhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpw ○ States of Matter Using a phase diagram to find a phase transition temperature or pressure Use the phase diagram of Substance X below to find the melting point of X when the pressure above the solid is 1.1 atm. pressure (atm) 16 08- solid liquid- 0 200 400 gas 600 temperature (K) Note: your answer must be within 25 °C of the exact answer to be graded correct. × 5arrow_forward
- S: Using a phase diagram leksogi/x/sl.exe/1ou-IgNs kr 7j8P3jH-IQs_pBan HhvTCeeBZbufuBYTI0Hz7m7D3ZdHYU+80XL-5alyVp O States of Matter Using a phase diagram to find a phase transition temperature or pressure se the phase diagram of Substance X below to find the boiling point of X when the pressure on the liquid is 1.6 atm. pressure (atm) 32- 16- solid liquid 0. gas 100 200 temperature (K) 300 Note: your answer must be within 12.5 °C of the exact answer to be graded correct. 10 Explanation Check § Q Search J 2025 McGraw Hill LLC. All Rights Researrow_forward151.2 254.8 85.9 199.6 241.4 87.6 242.5 186.4 155.8 257.1 242.9 253.3 256.0 216.6 108.7 239.0 149.7 236.4 152.1 222.7 148.7 278.2 268.7 234.4 262.7 283.2 143.6 QUESTION: Using this group of data on salt reduced tomato sauce concentration readings answer the following questions: 1. 95% Cl Confidence Interval (mmol/L) 2. [Na+] (mg/100 mL) 3. 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardResults Search Results Best Free Coursehero Unloc xb Success Confirmation of Q x O Google Pas alekscgi/x/lsl.exe/1o_u-IgNslkr 7j8P3jH-IQs_pBanHhvlTCeeBZbufu BYTI0Hz7m7D3ZcHYUt80XL-5alyVpwDXM TEZayFYCavJ17dZtpxbFD0Qggd1J O States of Matter Using a phase diagram to find a phase transition temperature or pressure Gabr 3/5 he pressure above a pure sample of solid Substance X at 101. °C is lowered. At what pressure will the sample sublime? Use the phase diagram of X below to nd your answer. pressure (atm) 24- 12 solid liquid gas 200 400 temperature (K) 600 ote: your answer must be within 0.15 atm of the exact answer to be graded correct. atm Thanation Check © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center I Q Search L³ ملةarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License