
(a)
Interpretation:
The electron-rich sites and electron-poor sites in the given elementary steps are to be identified.
Concept introduction:
An atom with partial or full negative charge is an electron-rich site whereas an atom with partial or full positive charge is an electron-poor site. In an elementary step, electrons tend to flow from an electron-rich site to an electron-poor site.
Answer to Problem 7.54P
The electron-rich and electron-poor sites for first elementary step are:
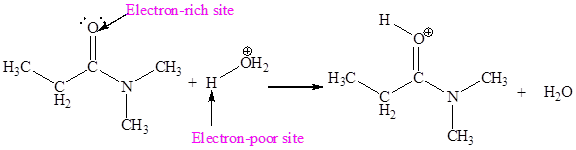
The electron-rich and electron-poor sites for second elementary step are:
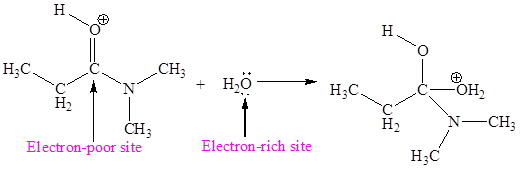
The electron-rich and electron-poor sites for third elementary step are:
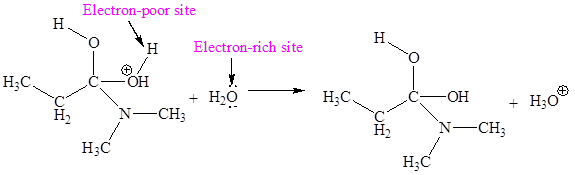
The electron-rich and electron-poor sites for fourth elementary step are:
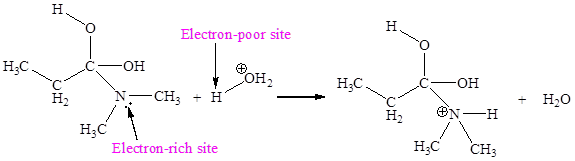
The electron-rich and electron-poor sites for fifth elementary step are:
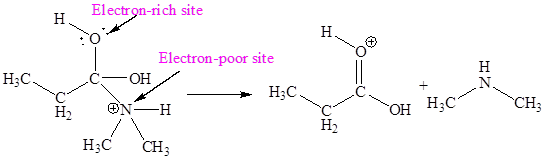
The electron-rich and electron-poor sites for sixth elementary step are:
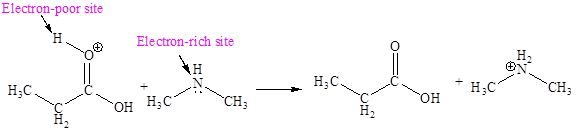
Explanation of Solution
The first elementary step is:
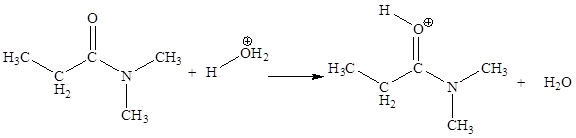
In the given elementary step, on the reactant side the oxygen atom which is a part carbonyl group having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
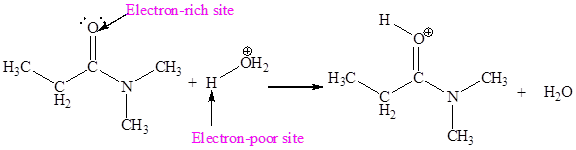
The second elementary step is:
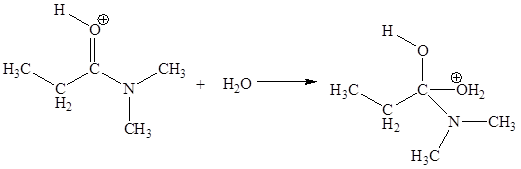
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The carbon atom adjacent to positively charged oxygen is electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
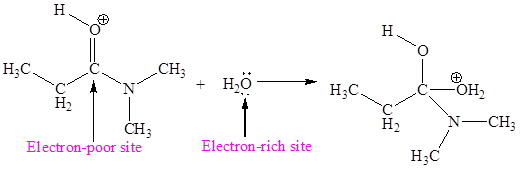
The third elementary step is:
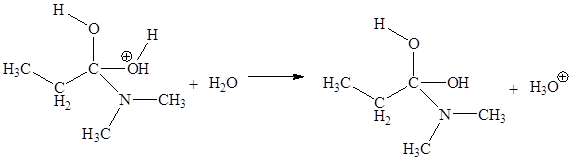
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The H atom adjacent to positively charged oxygen is electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
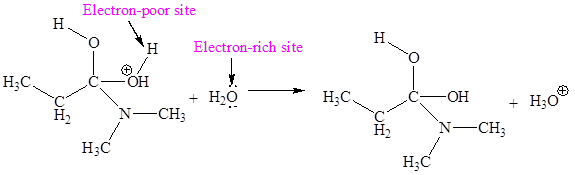
The fourth elementary step is:
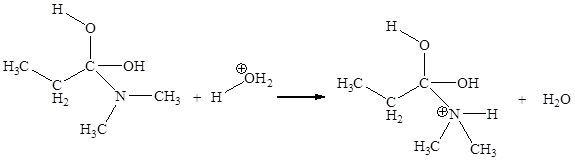
In the given elementary step, on the reactant side the nitrogen atom having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
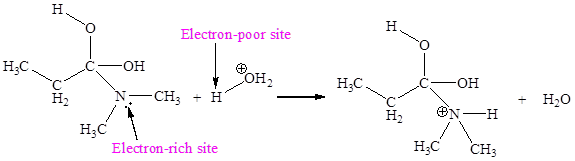
The fifth elementary step is:
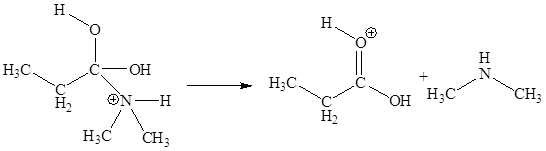
In the given elementary step, on the reactant side the oxygen atom of C-O bond having lone pairs is the electron-rich site. The nitrogen atom which is positively charged is the electron-poor site. The electron-rich and electron-poor sites for this step are labeled below:
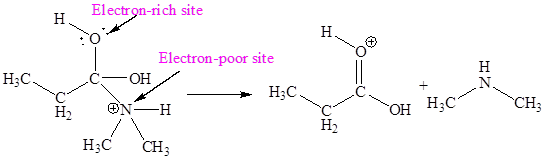
The sixth elementary step is:
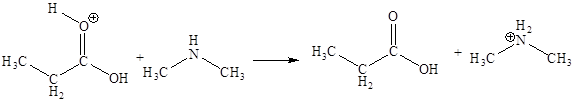
In the given elementary step, on the reactant side the hydrogen atom adjacent to positively charged oxygen atom is the electron-poor site. The nitrogen atom which is having lone pair of electrons is the electron-rich site. The electron-rich and electron-poor sites for this step are labeled below:
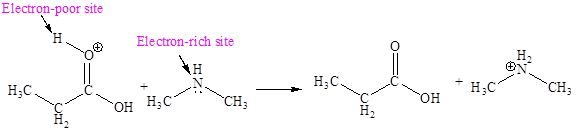
The electron-rich site and the electron-poor sites in each elementary step are identified on the basis of the negative and partial positive charge on respective atoms.
(b)
Interpretation:
In each of the given elementary steps the appropriate curved arrows are to be drawn.
Concept introduction:
The curved arrow can draw from electron rich site to an electron poor site to show the flow of electron from electron-rich site to electron-poor site. The first curved arrow drawn from the lone pair of negatively charged atom of electron-rich site to the less electronegative atom of electron-poor site. The second curved arrow drawn from the region between the less electronegative atom and more electronegative atom towards the more electronegative atom indicating the breaking of bond.
Answer to Problem 7.54P
The curved arrow mechanism for the first step is:
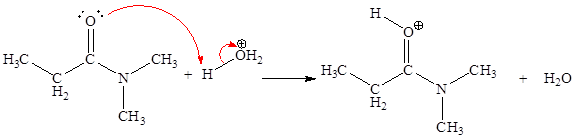
The curved arrow mechanism for the second step is:
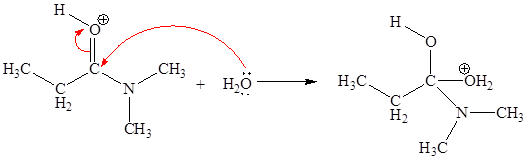
The curved arrow mechanism for the third step is:
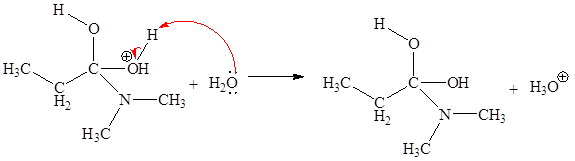
The curved arrow mechanism for the fourth step is:
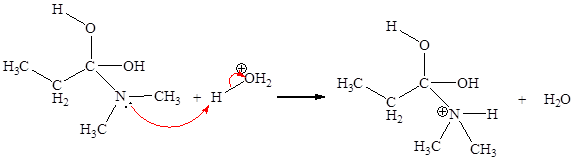
The curved arrow mechanism for the fifth step is:
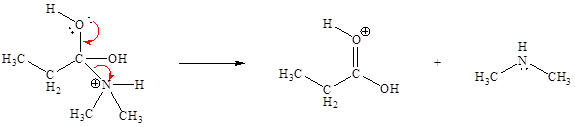
The curved arrow mechanism for the sixth step is:
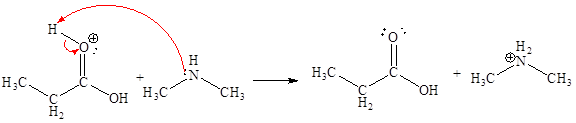
Explanation of Solution
The first elementary step is:
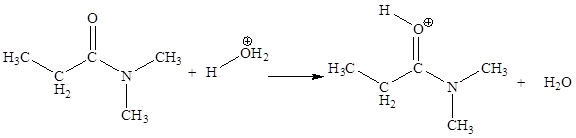
In above step the oxygen is an electron-rich site which attack at hydrogen atom bonded to positively charged oxygen atom is an electron poor site.
The curved arrow mechanism for this step is shown below:
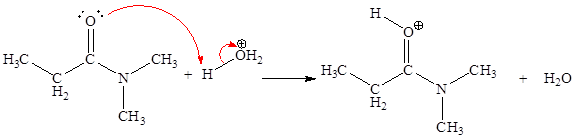
The first curved arrow is drawn from the lone pair of electron-rich oxygen to the electron-poor hydrogen atom representing the formation of
The second elementary step is:
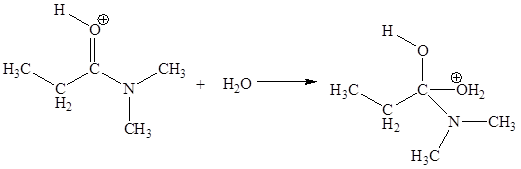
In this elementary step, the oxygen atom of water molecule having lone pair is the electron-rich site and the carbon atom adjacent to positively charged oxygen atom is electron-poor site. The curved arrow mechanism for this step is shown below:
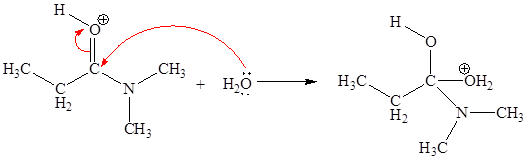
The first curved arrow is drawn from the lone pair of electron-rich oxygen atom to the electron-poor carbon atom representing the formation of new
The third elementary step is:
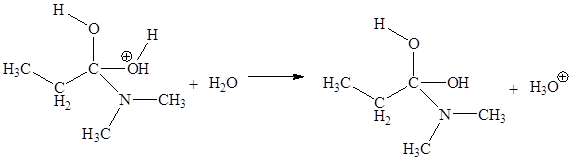
In this elementary step, the oxygen atom of water molecule with lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is an electron-poor site. The curved arrow mechanism for this step is shown below:

The first curved arrow is drawn from the lone pair of electron-rich oxygen atom of water to the electron-poor hydrogen atom representing the formation of new
The fourth elementary step is:
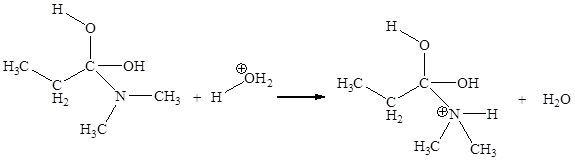
In the given elementary step, on the reactant side the nitrogen atom having lone pairs is the electron-rich site. The hydrogen atom bonded to positively charged oxygen is the electron-poor site. The curved arrow mechanism for this step is shown below:
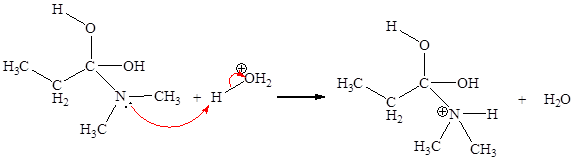
The first curved arrow is drawn from the lone pair of electron-rich nitrogen to the electron-poor hydrogen atom representing the formation of
The fifth elementary step is:
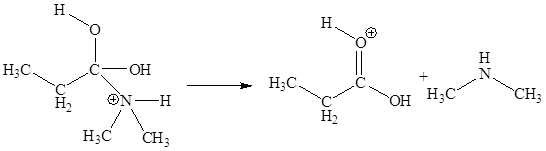
In the given elementary step, on the reactant side the oxygen atom of C-O bond having lone pairs is the electron-rich site. The nitrogen atom which is positively charged is the electron-poor site. The curved arrow mechanism for this step is shown below: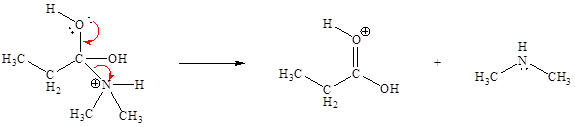
The first curved arrow is drawn from the lone pair of electron-rich oxygen to the
The sixth elementary step is:
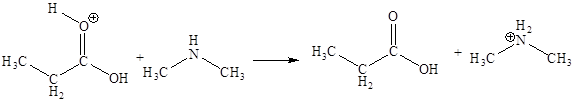
In the given elementary step, on the reactant side the hydrogen atom adjacent to positively charged oxygen atom is the electron-poor site. The nitrogen atom which is having lone pair of electrons is the electron-rich site. The curved arrow mechanism for this step is shown below:
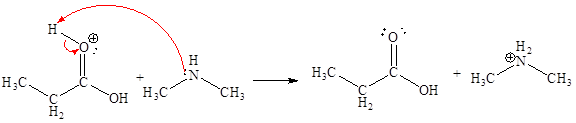
The first curved arrow is drawn from the lone pair of electron-rich nitrogen to the electron-poor hydrogen atom representing the formation of
The curved arrows for each of the given elementary steps are drawn from electron rich site to electron poor site and the less electronegative atom to more electronegative atom representing the formation and breaking of respective bonds.
(c)
Interpretation:
The names of each elementary step are to be identified.
Concept introduction:
In the nucleophilic addition step, the nucleophile forms a bond to the less electronegative atom and the
In a nucleophilic elimination step, a lone pair of electrons from a more electronegative atom forms a π bond to a less electronegative atom. A leaving group is simultaneously expelled to avoid exceeding an octet on the less electronegative atom.
An elementary step in which a proton is transferred from electron-poor site to electron- rich site and one bond is broken and another is formed simultaneously is called proton transfer step.
Answer to Problem 7.54P
The first elementary step is proton transfer reaction.
The nucleophilic addition reaction is the second elementary step.
The third elementary step is proton transfer reaction.
The fourth elementary step is proton transfer reaction.
The fifth elementary step is nucleophilic elimination reaction.
The sixth elementary step is proton transfer reaction.
Explanation of Solution
The first elementary step is:
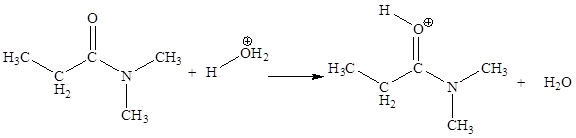
In the above elementary step, proton transferred from the positively charged oxygen of water molecule to the electron-rich oxygen atom. In this step, one
The second elementary step is:
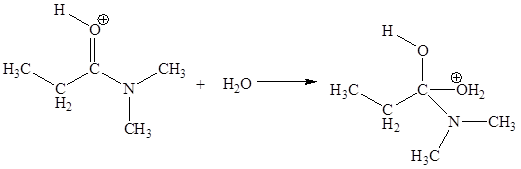
In the above elementary step, the oxygen atom of water forms a bond to the less electronegative carbon atom and the
The third elementary step is:
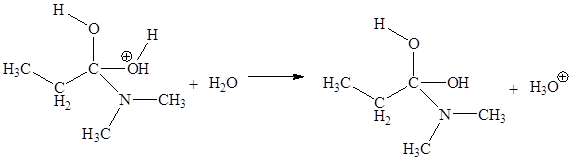
In the above elementary step, proton transferred from the positively charged oxygen of to the electron-rich oxygen atom of water molecule. In this step, one
The fourth elementary step is:
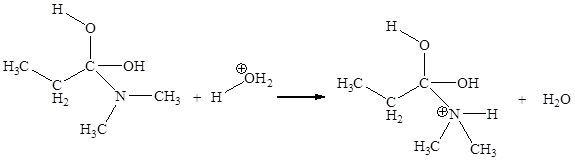
In the above elementary step, proton transferred from the positively charged oxygen of water molecule to the electron-rich nitrogen atom. In this step, one
The fifth elementary step is:
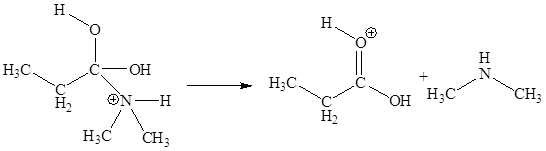
In the above elementary step, a lone pair of electrons from oxygen atom forms a π bond to a less electronegative carbon atom. A leaving group is simultaneously expelled to avoid exceeding an octet on the less electronegative carbon atom. Therefore, the step is named as nucleophilic elimination step.
The sixth elementary step is:
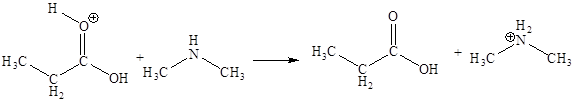
In the above elementary step, proton transferred from the positively charged oxygen of to the electron-rich nitrogen atom. In this step, one
The names for the given elementary steps are identified on the basis of type of bond forming and breaking.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Draw the complete mechanism for the acid-catalyzed hydration of this alkene. esc 田 Explanation Check 1 888 Q A slock Add/Remove step Q F4 F5 F6 A བྲA F7 $ % 5 @ 4 2 3 & 6 87 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce W E R T Y U S D LL G H IK DD 요 F8 F9 F10 F1 * ( 8 9 0 O P J K L Z X C V B N M H He commandarrow_forwardExplanation Check F1 H₂O H₂ Pd 1) MCPBA 2) H3O+ 1) Hg(OAc)2, H₂O 2) NaBH4 OH CI OH OH OH hydration halohydrin formation addition halogenation hydrogenation inhalation hydrogenation hydration ☐ halohydrin formation addition halogenation formation chelation hydrogenation halohydrin formation substitution hydration halogenation addition Ohalohydrin formation subtraction halogenation addition hydrogenation hydration F2 80 F3 σ F4 F5 F6 1 ! 2 # 3 $ 4 % 05 Q W & Å © 2025 McGraw Hill LLC. All Rights Reserved. F7 F8 ( 6 7 8 9 LU E R T Y U A F9arrow_forwardShow the mechanism steps to obtain the lowerenergy intermediate: *see imagearrow_forward
- Soap is made by the previous reaction *see image. The main difference between one soap and another soap isthe length (number of carbons) of the carboxylic acid. However, if a soap irritates your skin, they mostlikely used too much lye.Detergents have the same chemical structure as soaps except for the functional group. Detergentshave sulfate (R-SO4H) and phosphate (R-PO4H2) functional groups. Draw the above carboxylic acidcarbon chain but as the two variants of detergents. *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forwardWhat are the reactions or reagents used? *see imagearrow_forward
- Provide the mechanism for this transformation: *see imagearrow_forwardAssign all the signals individually (please assign the red, green and blue)arrow_forwardThe two pKa values of oxalic acid are 1.25 and 3.81. Why are they not the same value? Show the protontransfer as part of your explanation. *see imagearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


