
Concept explainers
(a)
Interpretation:
The energy difference between the two chair conformations of
Concept introduction:
The conformation of cyclohexane is a type of
Answer to Problem 7.14P
The energy difference between the two chair conformations of
Explanation of Solution
The two chair conformations of
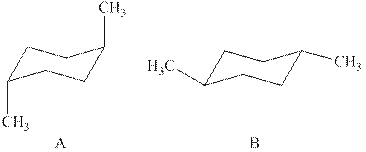
Figure 1
The
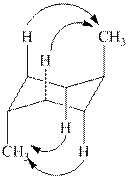
Figure 2
Thus, there are total four
The given value of contribution one
In case of chair conformation B, there is no
Therefore, the energy difference of between the two chair conformations of
Substitute the values of energies in above equation.
Thus, the energy difference is
The energy difference between the two chair conformations of the given compound is
(b)
Interpretation:
The energy difference between
Concept introduction:
The conformation of cyclohexane is a type of
Answer to Problem 7.14P
The energy difference between
Explanation of Solution
The chair conformations of
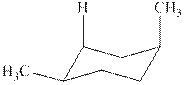
Figure 3
The
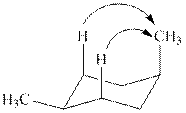
Figure 4
The given value of contribution one
The most stable chair conformation of
![]()
Figure 5
In case of most stable chair conformation of
Therefore, the energy difference of between the two chair conformations of
Substitute the values of energies in above equation.
Thus, the energy difference is
The energy difference between
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
- 7. Propene undergoes a hydration reaction with water in the presence of an acid. a. There are two possible products for this reaction, both with the formula C,H,O. Show their structural formulas and names. (A1, B2) SCH4UR Name: (answer for part a. here!) VER 3 2021-2022 b. Which of the two products do you predict will form. Explain your choice using details from your learning. (B3)arrow_forwardWhat are the major products of the following organic reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardWhat are the major products of the following reaction? Please provide a detailed explanation and a drawing to show how the reaction proceeds.arrow_forward
- What are the major products of the following organic reaction? Please include a detailed explanation as well as a drawing as to how the reaction proceeds.arrow_forwardPredict the organic product that forms in the reaction below: H + гон OH H+ H+ ☑ O Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the product. In the drawing area below, draw the skeletal ("line") structure of the missing organic product X. Explanation Check Click and drag to start drawing a structure. S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardIn the analysis of Mg content in a 25 mL sample, a titration volume of 5 mL was obtained using 0.01 M EDTA. Calculate the Mg content in the sample if the Ca content is 20 ppmarrow_forward
- Predict the organic products that form in the reaction below: H. H+ + OH H+ Y Note: You may assume you have an excess of either reactant if the reaction requires more than one of those molecules to form the products. In the drawing area below, draw the skeletal ("line") structures of the missing organic products X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. G X C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access +arrow_forward111 Carbonyl Chem Choosing reagants for a Wittig reaction What would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. × ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forwardA student proposes the transformation below in one step of an organic synthesis. There may be one or more reactants missing from the left-hand side, but there are no products missing from the right-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing reactants to the left-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + T X O O лет-ле HO OH HO OH This transformation can't be done in one step.arrow_forward
- Determine the structures of the missing organic molecules in the following reaction: X+H₂O H* H+ Y OH OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. X Sarrow_forwardPredict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. HO. O :☐ + G Na O.H Click and drag to start drawing a structure. XS xs H₂Oarrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H H C H- a -H b H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal groups may have slightly different sizes. a = b = 0 °arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

