
Organic Chemistry
2nd Edition
ISBN: 9781118452288
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 67IP
Interpretation Introduction
Interpretation: The mechanism for the given reaction should be identified.
Concept Introduction:
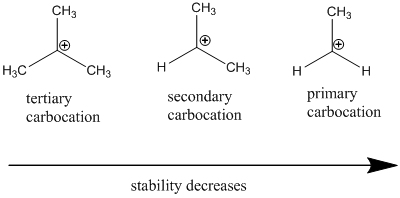
Carbocation: it is carbon ion that bears a positive charge on it.
Leaving group: it is a fragment that leaves from a substrate with a pair of electrons via heterolytic bond cleavage.
Carbocation stability order:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What would be the major product of the following reaction? Please include a detailed explanation of what is happening in this question. Include steps and a drawing to show this reaction proceeds and how the final product is formed. The correct answer is B. I put answer D and I don't really understand what is going on in the question.
What is the product of the following reaction? Please explain what is happening in this question. Provide a detailed explanation and a drawing showing how the reagent is reacting with the catalysts to product the correct product. The correct answer is B.
What is the missing intermediate 1 and the final product 2. Please include a detailed explanation explaining the steps of malonic ester synthesis. Please include drawings of the intermediate and how it occurs and how the final product is former.
Chapter 7 Solutions
Organic Chemistry
Ch. 7.2 - Prob. 1CCCh. 7.3 - Prob. 1LTSCh. 7.3 - Prob. 2PTSCh. 7.3 - Prob. 3PTSCh. 7.3 - Prob. 4ATSCh. 7.3 - Prob. 5ATSCh. 7.4 - Prob. 6CCCh. 7.4 - Prob. 2LTSCh. 7.4 - Prob. 7PTSCh. 7.4 - Prob. 8ATS
Ch. 7.4 - Prob. 3LTSCh. 7.4 - Prob. 9PTSCh. 7.4 - Prob. 10ATSCh. 7.4 - Prob. 11ATSCh. 7.4 - Prob. 12CCCh. 7.5 - Prob. 13CCCh. 7.5 - Prob. 4LTSCh. 7.5 - Prob. 14PTSCh. 7.5 - Prob. 15ATSCh. 7.5 - Prob. 5LTSCh. 7.5 - Prob. 16PTSCh. 7.5 - Prob. 17ATSCh. 7.6 - Prob. 18CCCh. 7.6 - Prob. 19CCCh. 7.6 - Prob. 20CCCh. 7.6 - Prob. 6LTSCh. 7.6 - Prob. 21PTSCh. 7.6 - Prob. 22ATSCh. 7.6 - Prob. 23ATSCh. 7.7 - Prob. 7LTSCh. 7.7 - Prob. 24PTSCh. 7.7 - Prob. 25ATSCh. 7.8 - Prob. 26CCCh. 7.8 - Prob. 27CCCh. 7.8 - Prob. 28CCCh. 7.8 - Prob. 29CCCh. 7.8 - Prob. 30CCCh. 7.8 - Prob. 8LTSCh. 7.8 - Prob. 31PTSCh. 7.8 - Prob. 32ATSCh. 7.9 - Prob. 9LTSCh. 7.9 - Prob. 33PTSCh. 7.9 - Prob. 34ATSCh. 7.9 - Prob. 35CCCh. 7 - Prob. 36PPCh. 7 - Prob. 37PPCh. 7 - Prob. 38PPCh. 7 - Prob. 39PPCh. 7 - Prob. 40PPCh. 7 - Prob. 41PPCh. 7 - Prob. 42PPCh. 7 - Prob. 43PPCh. 7 - Prob. 44PPCh. 7 - Prob. 45PPCh. 7 - Prob. 46PPCh. 7 - Prob. 47PPCh. 7 - Prob. 48PPCh. 7 - Prob. 49PPCh. 7 - Prob. 50PPCh. 7 - Prob. 51PPCh. 7 - Prob. 52PPCh. 7 - Prob. 53PPCh. 7 - Prob. 54PPCh. 7 - Prob. 55PPCh. 7 - Prob. 56PPCh. 7 - Prob. 57PPCh. 7 - Prob. 58PPCh. 7 - Prob. 59PPCh. 7 - Prob. 60PPCh. 7 - Prob. 61PPCh. 7 - Prob. 62PPCh. 7 - Prob. 63PPCh. 7 - Prob. 64IPCh. 7 - Prob. 65IPCh. 7 - Prob. 66IPCh. 7 - Prob. 67IPCh. 7 - Prob. 68IPCh. 7 - Prob. 69IPCh. 7 - Prob. 70IPCh. 7 - Prob. 71IPCh. 7 - Prob. 72IPCh. 7 - Prob. 73IPCh. 7 - Prob. 74IPCh. 7 - Prob. 75IPCh. 7 - Prob. 76IP
Knowledge Booster
Similar questions
- What would be the reagents and conditions above and below the arrow that will complete the proposed acetoacetic ester synthesis? If it cannot be done efficiently, then I will choose that answer. There could be 2 or 4 reagents involved. Please provide a detailed explanation and drawings showing how it would proceed with the correct reagents.arrow_forwardFor benzene, the ∆H° of vaporization is 30.72 kJ/mol and the ∆S° of vaporization is 86.97 J/mol・K. At 1.00 atm and 228.0 K, what is the ∆G° of vaporization for benzene, in kJ/mol?arrow_forwardThe reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reaction. it is spontaneous only at High T, it is spontaneous at low T it is nonspontaneous at all T it is spontanrous at all T. it is non spontaneous only at low T.arrow_forward
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY