
(a)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,
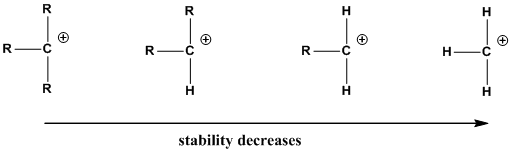
(b)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

(c)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

(d)
Interpretation: The carbocation intermediate and the type of carbocation present in the given substrates should be identified.
Concept introduction:
Carbocation intermediate: It is a fragment from the molecule in which the carbon atom bears a positive charge on it. The stability of the carbocation present in a molecule depends on the neighboring atom present in the molecule with respect to its resonance effects.
Resonance: The delocalization of pi electrons or lone pair of electrons present in p orbital within a molecule. The molecule is said to be more stable if it is resonance stabilized.
The order of the stability of carbocation,

Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





