
PRINCIPLES OF MODERN CHEMISTRY-OWLV2
8th Edition
ISBN: 9781305271609
Author: OXTOBY
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 48CP
Interpretation Introduction
Interpretation: Whether the wavelength of maximum absorption in naphthalene is shorter or longer than 255 nm that is maximum absorption in benzene needs to be determined.
Concept Introduction:Benzene shows resonance due to delocalization of
electrons. The naphthalene is formed by fusion of two benzene molecules. The structure of benzene and naphthalene is represented as follows:
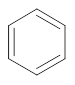 and
and 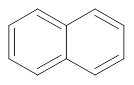
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3-Oxo-butanenitrile and (E)-2-butenal are mixed with sodium ethoxide in ethanol. Draw and name the structures of the products.
What is the reason of the following(use equations if possible)
a.) In MO preperation through diazotization: Addition of sodium nitrite in acidfied solution in order to form diazonium salt
b.) in MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at low pH
c.) In MO experiment: addition of sodium hydroxide solution in the last step to isolate the product MO. What is the color of MO at pH 4.5
d.) Avoiding not cooling down the reaction mixture when preparing the diazonium salt
e.) Cbvc
A 0.552-g sample of an unknown acid was dissolved in water to a total volume of 20.0 mL. This sample was titrated with 0.1103 M KOH. The equivalence point occurred at 29.42 mL base added. The pH of the solution at 10.0 mL base added was 3.72.
Determine the molar mass of the acid.
Determine the Ka of the acid.
Chapter 7 Solutions
PRINCIPLES OF MODERN CHEMISTRY-OWLV2
Ch. 7 - Prob. 1PCh. 7 - Is it possible for a motor fuel to have a negative...Ch. 7 - A gaseous alkane is burned completely in oxygen....Ch. 7 - A gaseous alkyne is burned completely in oxygen....Ch. 7 - Write a chemical equation involving structural...Ch. 7 - Prob. 6PCh. 7 - Prob. 7PCh. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10P
Ch. 7 - Prob. 11PCh. 7 - Prob. 12PCh. 7 - Prob. 13PCh. 7 - State the hybridization of each of the carbon...Ch. 7 - Prob. 15PCh. 7 - Prob. 16PCh. 7 - Prob. 17PCh. 7 - In a recent year, the United States produced...Ch. 7 - Prob. 19PCh. 7 - Prob. 20PCh. 7 - Prob. 21PCh. 7 - Prob. 22PCh. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - Prob. 25PCh. 7 - Prob. 26PCh. 7 - Acetic acid can be made by the oxidation of...Ch. 7 - Acrylic fibers are polymers made from a starting...Ch. 7 - Compare the bonding in formic acid (HCOOH) with...Ch. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Prob. 32PCh. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Describe the changes in hydrocarbon structure and...Ch. 7 - trans-Cyclodecene boils at 193C, but...Ch. 7 - Prob. 38APCh. 7 - Prob. 39APCh. 7 - Consider the following proposed structures for...Ch. 7 - Prob. 41APCh. 7 - Prob. 42APCh. 7 - Prob. 43APCh. 7 - Prob. 44APCh. 7 - Prob. 45APCh. 7 - The steroid stanolone is an androgenic steroid (a...Ch. 7 - The structure of the molecule cyclohexene is Does...Ch. 7 - Prob. 48CP
Knowledge Booster
Similar questions
- As the lead product manager at OrganometALEKS Industries, you are trying to decide if the following reaction will its major product: 2,0° with a new C-C bond as If this reaction will work, draw the major organic product or products you would expect in the drawing aree below. If there's more than one major product, you can draw them in any arrangement you like. Be sure you use wedge and desh bonds if necessary, for example to distinguish between major products with different stereochemistry. If the major products of this reaction won't have a new C-C bond, just check the box under the drawing area and leave it blank.arrow_forwardwrite the mechanism of the nucleophilic acyl substitution reaction, please give an examplearrow_forwardThe compound in the figure is reacted with 10 n-butyllihium, 2° propanone, and 3º H2O. Draw and name the products obtained. SiMe3arrow_forward
- Caffeine (C8H10N4O2, pictured below) is a weak base. The pKb of caffeine is 10.4. What is the pH of a 0.0155 M solution of caffeine?arrow_forward2-Cyclopentyl-2-methyl-1,3-dioxolane is reacted with H₂SO₄. Draw and name the structures of the products.arrow_forwardIndicate the products of the reaction of 1-cyclohexyl-2,2-dimethylpropan-1-one with CH3CO3H (). Draw the structures of the compounds.arrow_forward
- Write chemical equations for: the reaction of benzoic acid chloride with grignard reagent [CH3MgX] the reaction of butanoic acid with methyl amine [CH3NH2]arrow_forward2-(3-Aminopropyl)cyclohexan-1-one is reacted with H₂SO₄. Draw the structures of the products.arrow_forwardPlease help me solve number 2arrow_forward
- Choose the best reagents to complete the following reaction. 오 Na2Cr2O7 H2SO4, H2O Problem 22 of 35 A Na2Cr2O7 H2SO4, H2O H2/Pt B pressure OH 1. NaBH4 C 2. H3O+ D DMP (Dess-Martin Periodinane) CH2Cl2 CrO3 Done Dramabana_Minor Submitarrow_forwardIndicate the products of the reaction of Cycloheptanone with pyrrolidine (cat. H+). Draw the structures of the compounds.arrow_forwardIndicate the products of the reaction of 2-(3-aminopropyl)cyclohexan-1-one with H2SO4. Draw the structures of the compounds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning