
PRINCIPLES OF MODERN CHEMISTRY-OWLV2
8th Edition
ISBN: 9781305271609
Author: OXTOBY
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 47CP
The structure of the molecule cyclohexene is
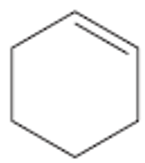
Does the absorption of ultraviolet light by cyclohexene occur at longer or at shorter wavelengths than the absorption by benzene? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
None
man Campus Depa
(a) Draw the three products (constitutional isomers) obtained when 2-methyl-3-hexene reacts with water and
a trace of H2SO4. Hint: one product forms as the result of a 1,2-hydride shift. (1.5 pts)
This is the acid-catalyzed alkene hydration reaction.
None
Chapter 7 Solutions
PRINCIPLES OF MODERN CHEMISTRY-OWLV2
Ch. 7 - Prob. 1PCh. 7 - Is it possible for a motor fuel to have a negative...Ch. 7 - A gaseous alkane is burned completely in oxygen....Ch. 7 - A gaseous alkyne is burned completely in oxygen....Ch. 7 - Write a chemical equation involving structural...Ch. 7 - Prob. 6PCh. 7 - Prob. 7PCh. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10P
Ch. 7 - Prob. 11PCh. 7 - Prob. 12PCh. 7 - Prob. 13PCh. 7 - State the hybridization of each of the carbon...Ch. 7 - Prob. 15PCh. 7 - Prob. 16PCh. 7 - Prob. 17PCh. 7 - In a recent year, the United States produced...Ch. 7 - Prob. 19PCh. 7 - Prob. 20PCh. 7 - Prob. 21PCh. 7 - Prob. 22PCh. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - Prob. 25PCh. 7 - Prob. 26PCh. 7 - Acetic acid can be made by the oxidation of...Ch. 7 - Acrylic fibers are polymers made from a starting...Ch. 7 - Compare the bonding in formic acid (HCOOH) with...Ch. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Prob. 32PCh. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Describe the changes in hydrocarbon structure and...Ch. 7 - trans-Cyclodecene boils at 193C, but...Ch. 7 - Prob. 38APCh. 7 - Prob. 39APCh. 7 - Consider the following proposed structures for...Ch. 7 - Prob. 41APCh. 7 - Prob. 42APCh. 7 - Prob. 43APCh. 7 - Prob. 44APCh. 7 - Prob. 45APCh. 7 - The steroid stanolone is an androgenic steroid (a...Ch. 7 - The structure of the molecule cyclohexene is Does...Ch. 7 - Prob. 48CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H HgSO4, H2O H2SO4arrow_forward12. Choose the best diene and dienophile pair that would react the fastest. CN CN CO₂Et -CO₂Et .CO₂Et H3CO CO₂Et A B C D E Farrow_forward(6 pts - 2 pts each part) Although we focused our discussion on hydrogen light emission, all elements have distinctive emission spectra. Sodium (Na) is famous for its spectrum being dominated by two yellow emission lines at 589.0 and 589.6 nm, respectively. These lines result from electrons relaxing to the 3s subshell. a. What is the photon energy (in J) for one of these emission lines? Show your work. b. To what electronic transition in hydrogen is this photon energy closest to? Justify your answer-you shouldn't need to do numerical calculations. c. Consider the 3s subshell energy for Na - use 0 eV as the reference point for n=∞. What is the energy of the subshell that the electron relaxes from? Choose the same emission line that you did for part (a) and show your work.arrow_forward
- Nonearrow_forward(9 Pts) In one of the two Rare Earth element rows of the periodic table, identify an exception to the general ionization energy (IE) trend. For the two elements involved, answer the following questions. Be sure to cite sources for all physical data that you use. a. (2 pts) Identify the two elements and write their electronic configurations. b. (2 pts) Based on their configurations, propose a reason for the IE trend exception. c. (5 pts) Calculate effective nuclear charges for the last electron in each element and the Allred-Rochow electronegativity values for the two elements. Can any of these values explain the IE trend exception? Explain how (not) - include a description of how IE relates to electronegativity.arrow_forwardPlease explain thoroughly and provide steps to draw.arrow_forward
- As you can see in the picture, the instrument uses a Xe source. Given that the instrument is capable of measuring from 200-800nm, if Xe was not used, what other source(s) could be used? Refer to figure 7-3. How many monochrometers does this instrument have? Why? Trace the light as it goes from the Xenon lamp all the way to the circle just slightly to the right and a little bit down from S4. What do you think that circle is? In class we talked about many types of these, which kind do you think this one is for a fluorimeter? Why? Explain. What is/are some strategy(ies) that this instrument has for dealing with noise that you see present in the optics diagram? Why does a fluorescence cuvette have to be clear on four sides?arrow_forwardProvide steps and thoroughly solve.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY