
Concept explainers
(a1)
To explain: The basis of the procedure for determining the number of
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a1)
Explanation of Solution
The carbon number 6 of glucose residue present in
Pictorial representation: Fig 1: represents the structure of 2,3-di-O-methylglucose.
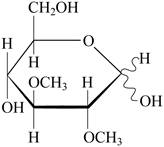
Fig 1: Structure of 2,3-di-O-methylglucose.
(a2)
To explain: What happens to the unbranched glucose residues in amylopectin during the methylation and hydrolysis procedure.
Introduction:
Carbohydrates are present in different forms. The carbohydrate made up of only one kind of sugar is monosaccharide, if formed of two types of sugars it is called disaccharide and if made up of many types of sugar it is polysaccharide.
(a2)
Explanation of Solution
The polysaccharides are large
In case of unbranched residue, methylation and hydrolysis process would yield 2,3,6-tri-O-methylglucose, as the hydroxyl group at carbon 2, 3, and 6 are free.
(b)
To calculate: The percentage of glucose residues in amylopectin contained
Introduction:
The polysaccharides are large polymer molecules composed of monosaccharides unit. Starch is an example of polysaccharide that are made up of amylose and amylopectin molecule. Amylose is a linear chain of starch that are compose of glucose units. Each glucose unit in amylose are joined through α-glycosidic bond. Whereas, amylopectin is branched structure of starch that contain β-glycosidic bond.
Given: 258 mg or
(b)
Explanation of Solution
The average molecular weight of glucose is 162g/mol, then
Thus, the total weight of amylopectin in
The weight of branched residue of 2,3-di-O-methylglucose in
Thus, the average molecular weight of glucose in 2,3-di-O-methylglucose is
The percentage of glucose residue glucose residue present in amylopectin having
The weight of branched residue is
Now, substitute the value in the above given equation and calculate the percentage of glucose residue in amylopectin.
Thus, the percentage of glucose residue in amylopectin having
The percentage of glucose residue in amylopectin is 3.75 %.
Want to see more full solutions like this?
Chapter 7 Solutions
Loose-leaf Version for Lehninger Principles of Biochemistry 7E & SaplingPlus for Lehninger Principles of Biochemistry 7E (Six-Month Access)
- Which type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward+NH+ CO₂ +P H₂N + ATP H₂N NH₂ +ADParrow_forwardWhich type of enzyme catalyses the following reaction? oxidoreductase, transferase, hydrolase, lyase, isomerase, or ligase.arrow_forward
- Which features of the curves in Figure 30-2 indicates that the enzyme is not consumed in the overall reaction? ES is lower in energy that E + S and EP is lower in energy than E + P. What does this tell you about the stability of ES versus E + S and EP versus E + P.arrow_forwardLooking at the figure 30-5 what intermolecular forces are present between the substrate and the enzyme and the substrate and cofactors.arrow_forwardprovide short answers to the followings Urgent!arrow_forward
- Pyruvate is accepted into the TCA cycle by a “feeder” reaction using the pyruvatedehydrogenase complex, resulting in acetyl-CoA and CO2. Provide a full mechanismfor this reaction utilizing the TPP cofactor. Include the roles of all cofactors.arrow_forwardB- Vitamins are converted readily into important metabolic cofactors. Deficiency inany one of them has serious side effects. a. The disease beriberi results from a vitamin B 1 (Thiamine) deficiency and ischaracterized by cardiac and neurological symptoms. One key diagnostic forthis disease is an increased level of pyruvate and α-ketoglutarate in thebloodstream. How does this vitamin deficiency lead to increased serumlevels of these factors? b. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 5 deficiency? c. What would you expect the effect on the TCA intermediates for a patientsuffering from vitamin B 2 /B 3 deficiency?arrow_forwardDraw the Krebs Cycle and show the entry points for the amino acids Alanine,Glutamic Acid, Asparagine, and Valine into the Krebs Cycle - (Draw the Mechanism). How many rounds of Krebs will be required to waste all Carbons of Glutamic Acidas CO2?arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





