
Bundle: Principles of Physics: A Calculus-Based Text, 5th + WebAssign Printed Access Card for Serway/Jewett's Principles of Physics: A Calculus-Based Text, 5th Edition, Multi-Term
5th Edition
ISBN: 9781133422013
Author: Raymond A. Serway; John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 21P
A 5.00-kg block is set into motion up an inclined plane with an initial speed of υi = 8.00 m/s (Fig. P7.21). The block comes to rest after traveling d = 3.00 m along the plane, which is inclined at an angle of θ = 30.0° to the horizontal. For this motion, determine (a) the change in the block’s kinetic energy, (b) the change in the potential energy of the block-Earth system, and (c) the friction force exerted on the block (assumed to be constant), (d) What is the coefficient of kinetic friction?
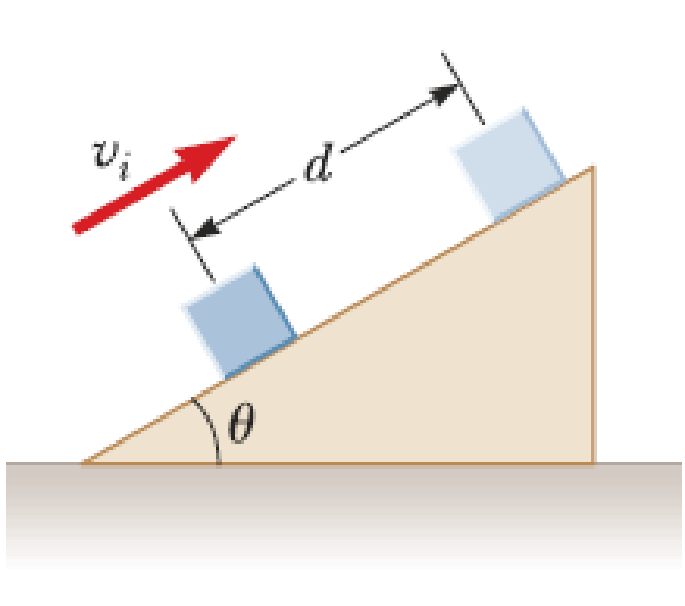
Figure P7.21
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A cylinder with a piston contains 0.153 mol of
nitrogen at a pressure of 1.83×105 Pa and a
temperature of 290 K. The nitrogen may be
treated as an ideal gas. The gas is first compressed
isobarically to half its original volume. It then
expands adiabatically back to its original volume,
and finally it is heated isochorically to its original
pressure.
Part A
Compute the temperature at the beginning of the adiabatic expansion.
Express your answer in kelvins.
ΕΠΙ ΑΣΦ
T₁ =
?
K
Submit
Request Answer
Part B
Compute the temperature at the end of the adiabatic expansion.
Express your answer in kelvins.
Π ΑΣΦ
T₂ =
Submit
Request Answer
Part C
Compute the minimum pressure.
Express your answer in pascals.
ΕΠΙ ΑΣΦ
P =
Submit
Request Answer
?
?
K
Pa
Learning Goal:
To understand the meaning and the basic applications of
pV diagrams for an ideal gas.
As you know, the parameters of an ideal gas are
described by the equation
pV = nRT,
where p is the pressure of the gas, V is the volume of
the gas, n is the number of moles, R is the universal gas
constant, and T is the absolute temperature of the gas. It
follows that, for a portion of an ideal gas,
pV
= constant.
Τ
One can see that, if the amount of gas remains constant,
it is impossible to change just one parameter of the gas:
At least one more parameter would also change. For
instance, if the pressure of the gas is changed, we can
be sure that either the volume or the temperature of the
gas (or, maybe, both!) would also change.
To explore these changes, it is often convenient to draw a
graph showing one parameter as a function of the other.
Although there are many choices of axes, the most
common one is a plot of pressure as a function of
volume: a pV diagram.
In this problem, you…
Learning Goal:
To understand the meaning and the basic applications of
pV diagrams for an ideal gas.
As you know, the parameters of an ideal gas are
described by the equation
pV = nRT,
where p is the pressure of the gas, V is the volume of
the gas, n is the number of moles, R is the universal gas
constant, and T is the absolute temperature of the gas. It
follows that, for a portion of an ideal gas,
pV
= constant.
T
One can see that, if the amount of gas remains constant,
it is impossible to change just one parameter of the gas:
At least one more parameter would also change. For
instance, if the pressure of the gas is changed, we can
be sure that either the volume or the temperature of the
gas (or, maybe, both!) would also change.
To explore these changes, it is often convenient to draw a
graph showing one parameter as a function of the other.
Although there are many choices of axes, the most
common one is a plot of pressure as a function of
volume: a pV diagram.
In this problem, you…
Chapter 7 Solutions
Bundle: Principles of Physics: A Calculus-Based Text, 5th + WebAssign Printed Access Card for Serway/Jewett's Principles of Physics: A Calculus-Based Text, 5th Edition, Multi-Term
Ch. 7.1 - By what transfer mechanisms does energy enter and...Ch. 7.1 - Consider a block sliding over a horizontal surface...Ch. 7.2 - Prob. 7.3QQCh. 7.2 - Prob. 7.4QQCh. 7.4 - Prob. 7.5QQCh. 7 - You hold a slingshot at arms length, pull the...Ch. 7 - An athlete jumping vertically on a trampoline...Ch. 7 - Prob. 3OQCh. 7 - Two children stand on a platform at the top of a...Ch. 7 - Answer yes or no to each of the following...
Ch. 7 - A ball of clay falls freely to the hard floor. It...Ch. 7 - What average power is generated by a 70.0-kg...Ch. 7 - In a laboratory model of cars skidding to a stop,...Ch. 7 - At the bottom of an air track tilted at angle , a...Ch. 7 - One person drops a ball from the top of a building...Ch. 7 - Prob. 2CQCh. 7 - Does everything have energy? Give the reasoning...Ch. 7 - Prob. 4CQCh. 7 - Prob. 5CQCh. 7 - Prob. 6CQCh. 7 - A block is connected to a spring that is suspended...Ch. 7 - Consider the energy transfers and transformations...Ch. 7 - Prob. 9CQCh. 7 - Prob. 10CQCh. 7 - Prob. 1PCh. 7 - Prob. 2PCh. 7 - Review. A bead slides without friction around a...Ch. 7 - At 11:00 a.m, on September 7, 2001, more than one...Ch. 7 - A block of mass 0.250 kg is placed on top of a...Ch. 7 - A block of mass m = 5.00 kg is released from point...Ch. 7 - Two objects are connected by a light string...Ch. 7 - Prob. 8PCh. 7 - Prob. 9PCh. 7 - Prob. 10PCh. 7 - Prob. 11PCh. 7 - A crate of mass 10.0 kg is pulled up a rough...Ch. 7 - Prob. 13PCh. 7 - Prob. 14PCh. 7 - A block of mass m = 2.00 kg is attached to a...Ch. 7 - Prob. 16PCh. 7 - A smooth circular hoop with a radius of 0.500 m is...Ch. 7 - Prob. 18PCh. 7 - Prob. 19PCh. 7 - As shown in Figure P7.20, a green bead of mass 25...Ch. 7 - A 5.00-kg block is set into motion up an inclined...Ch. 7 - The coefficient of friction between the block of...Ch. 7 - Prob. 23PCh. 7 - Prob. 24PCh. 7 - Prob. 25PCh. 7 - Prob. 26PCh. 7 - A child of mass m starts from rest and slides...Ch. 7 - The electric motor of a model train accelerates...Ch. 7 - Prob. 29PCh. 7 - Prob. 30PCh. 7 - Prob. 31PCh. 7 - Sewage at a certain pumping station is raised...Ch. 7 - Prob. 33PCh. 7 - Prob. 34PCh. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Prob. 39PCh. 7 - Prob. 40PCh. 7 - A loaded ore car has a mass of 950 kg and rolls on...Ch. 7 - Prob. 42PCh. 7 - A certain automobile engine delivers 2.24 104 W...Ch. 7 - Prob. 44PCh. 7 - A small block of mass m = 200 g is released from...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - Prob. 48PCh. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - Prob. 51PCh. 7 - Prob. 52PCh. 7 - Jonathan is riding a bicycle and encounters a hill...Ch. 7 - Prob. 54PCh. 7 - A horizontal spring attached to a wall has a force...Ch. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Prob. 59PCh. 7 - Prob. 60PCh. 7 - Prob. 61PCh. 7 - Prob. 62PCh. 7 - Make an order-of-magnitude estimate of your power...Ch. 7 - Prob. 64PCh. 7 - Prob. 65PCh. 7 - Review. As a prank, someone has balanced a pumpkin...Ch. 7 - Review. The mass of a car is 1 500 kg. The shape...Ch. 7 - A 1.00-kg object slides to the right on a surface...Ch. 7 - A childs pogo stick (Fig. P7.69) stores energy in...Ch. 7 - Prob. 70PCh. 7 - Prob. 71PCh. 7 - Prob. 72PCh. 7 - A block of mass m1 = 20.0 kg is connected to a...Ch. 7 - Prob. 74PCh. 7 - Prob. 75PCh. 7 - Prob. 76PCh. 7 - Prob. 77PCh. 7 - Prob. 78PCh. 7 - A block of mass 0.500 kg is pushed against a...Ch. 7 - A pendulum, comprising a light string of length L...Ch. 7 - Jane, whose mass is 50.0 kg, needs to swing across...Ch. 7 - A roller-coaster car shown in Figure P7.82 is...Ch. 7 - Prob. 83P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- ■ Review | Constants A cylinder with a movable piston contains 3.75 mol of N2 gas (assumed to behave like an ideal gas). Part A The N2 is heated at constant volume until 1553 J of heat have been added. Calculate the change in temperature. ΜΕ ΑΣΦ AT = Submit Request Answer Part B ? K Suppose the same amount of heat is added to the N2, but this time the gas is allowed to expand while remaining at constant pressure. Calculate the temperature change. AT = Π ΑΣΦ Submit Request Answer Provide Feedback ? K Nextarrow_forward4. I've assembled the following assortment of point charges (-4 μC, +6 μC, and +3 μC) into a rectangle, bringing them together from an initial situation where they were all an infinite distance away from each other. Find the electric potential at point "A" (marked by the X) and tell me how much work it would require to bring a +10.0 μC charge to point A if it started an infinite distance away (assume that the other three charges remains fixed). 300 mm -4 UC "A" 0.400 mm +6 UC +3 UC 5. It's Friday night, and you've got big party plans. What will you do? Why, make a capacitor, of course! You use aluminum foil as the plates, and since a standard roll of aluminum foil is 30.5 cm wide you make the plates of your capacitor each 30.5 cm by 30.5 cm. You separate the plates with regular paper, which has a thickness of 0.125 mm and a dielectric constant of 3.7. What is the capacitance of your capacitor? If you connect it to a 12 V battery, how much charge is stored on either plate? =arrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV T = constant. One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forward
- A-e pleasearrow_forwardTwo moles of carbon monoxide (CO) start at a pressure of 1.4 atm and a volume of 35 liters. The gas is then compressed adiabatically to 1/3 this volume. Assume that the gas may be treated as ideal. Part A What is the change in the internal energy of the gas? Express your answer using two significant figures. ΕΠΙ ΑΣΦ AU = Submit Request Answer Part B Does the internal energy increase or decrease? internal energy increases internal energy decreases Submit Request Answer Part C ? J Does the temperature of the gas increase or decrease during this process? temperature of the gas increases temperature of the gas decreases Submit Request Answerarrow_forwardYour answer is partially correct. Two small objects, A and B, are fixed in place and separated by 2.98 cm in a vacuum. Object A has a charge of +0.776 μC, and object B has a charge of -0.776 μC. How many electrons must be removed from A and put onto B to make the electrostatic force that acts on each object an attractive force whose magnitude is 12.4 N? e (mea is the es a co le E o ussian Number Tevtheel ed Media ! Units No units → answe Tr2Earrow_forward
- 4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forward4 Problem 4) A particle is being pushed up a smooth slot by a rod. At the instant when 0 = rad, the angular speed of the arm is ė = 1 rad/sec, and the angular acceleration is = 2 rad/sec². What is the net force acting on the 1 kg particle at this instant? Express your answer as a vector in cylindrical coordinates. Hint: You can express the radial coordinate as a function of the angle by observing a right triangle. (20 pts) Ꮎ 2 m Figure 3: Particle pushed by rod along vertical path.arrow_forwardplease solve and answer the question correctly. Thank you!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Kinetic Energy and Potential Energy; Author: Professor Dave explains;https://www.youtube.com/watch?v=g7u6pIfUVy4;License: Standard YouTube License, CC-BY