
Concept explainers
(a)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
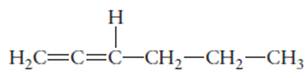
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
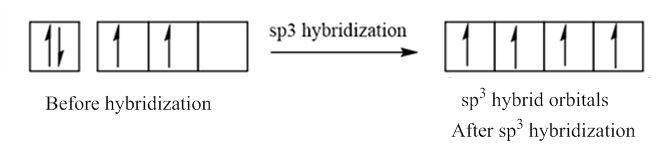
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(b)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
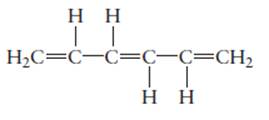
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
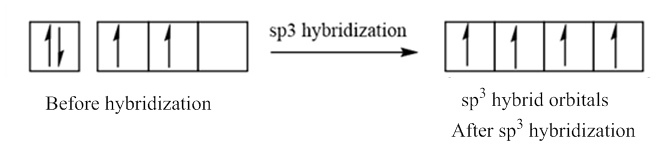
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(c)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
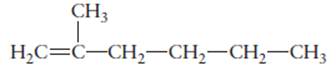
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
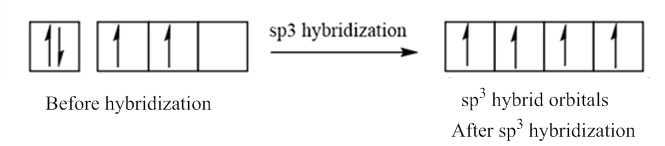
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
(d)
Interpretation:
The hybridization of each carbon atom in following structure should be determined.
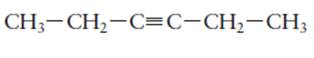
Concept Introduction:
The process of the mixing of orbitals having similar symmetry and energy to results equal number of orbitals is known as hybridization.
The formed orbitals are known as hybrid orbitals.
Carbon belongs to group 4 of the periodic table and its atomic number is 6.
The electronic configuration of carbon is:
In the hybridization, carbon uses 2s and 2p orbitals.
For example:
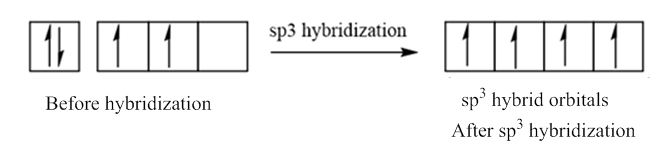
In case of sp3 hybridization, one 2s and three 2p orbitals of carbon combine to form four sp3 hybrid orbitals.
The electronic configuration of carbon before and after sp3 hybridization is:
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Bundle: Principles of Modern Chemistry, Loose-leaf Version, 8th + LMS Integrated for OWLv2 with MindTap Reader, 4 terms (24 months) Printed Access Card
- 7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward7 Comment on the general features of the predicted (extremely simplified) ¹H- NMR spectrum of lycopene that is provided below. 00 6 57 PPM 3 2 1 0arrow_forwardIndicate the compound formula: dimethyl iodide (propyl) sulfonium.arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStaxChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





