
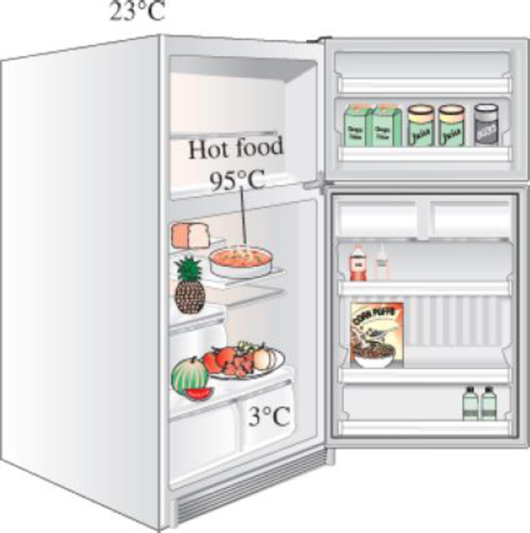
It is commonly recommended that hot foods be cooled first to room temperature by simply waiting a while before they are put into the refrigerator to save energy. Despite this commonsense recommendation, a person keeps cooking a large pan of stew three times a week and putting the pan into the refrigerator while it is still hot, thinking that the money saved is probably too little. But he says he can be convinced if you can show that the money saved is significant. The average mass of the pan and its contents is 5 kg. The average temperature of the kitchen is 23°C, and the average temperature of the food is 95°C when it is taken off the stove. The refrigerated space is maintained at 3°C, and the average specific heat of the food and the pan can be taken to be 3.9 kJ/kg·°C. If the refrigerator has a coefficient of performance of 1.5 and the cost of electricity is $0.125/kWh, determine how much this person will save a year by waiting for the food to cool to room temperature before putting it into the refrigerator.
FIGURE P6–123
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
CONNECT FOR THERMODYNAMICS: AN ENGINEERI
- 20. [Ans. 9; 71.8 mm] A semi-elliptical laminated spring is made of 50 mm wide and 3 mm thick plates. The length between the supports is 650 mm and the width of the band is 60 mm. The spring has two full length leaves and five graduated leaves. If the spring carries a central load of 1600 N, find: 1. Maximum stress in full length and graduated leaves for an initial condition of no stress in the leaves. 2. The maximum stress if the initial stress is provided to cause equal stress when loaded. [Ans. 590 MPa ; 390 MPa ; 450 MPa ; 54 mm] 3. The deflection in parts (1) and (2).arrow_forwardQ6/ A helical square section spring is set inside another, the outer spring having a free length of 35 mm greater than the inner spring. The dimensions of each spring are as follows: Mean diameter (mm) Side of square section (mm) Active turns Outer Inner Spring Spring 120 70 8 7 20 15 Determine the (1) Maximum deflection of the two springs and (2) Equivalent spring rate of the two springs after sufficient load has been applied to deflect the outer spring 60 mm. Use G = 83 GN/m².arrow_forwardQ2/ The bumper springs of a railway carriage are to be made of rectangular section wire. The ratio of the longer side of the wire to its shorter side is 1.5, and the ratio of mean diameter of spring to the longer side of wire is nearly equal to 6. Three such springs are required to bring to rest a carriage weighing 25 kN moving with a velocity of 75 m/min with a maximum deflection of 200 mm. Determine the sides of the rectangular section of the wire and the mean diameter of coils when the shorter side is parallel to the axis of the spring. The allowable shear stress is not to exceed 300 MPa and G = 84 kN/mm². Q6/ A belicalarrow_forward
- 11. A load of 2 kN is dropped axially on a close coiled helical spring, from a height of 250 mm. The spring has 20 effective turns, and it is made of 25 mm diameter wire. The spring index is 8. Find the maximum shear stress induced in the spring and the amount of compression produced. The modulus of rigidity for the material of the spring wire is 84 kN/mm². [Ans. 287 MPa; 290 mm]arrow_forwardWhat is the reason for this composition?arrow_forwardHomework: ANOVA Table for followed design B AB Dr -1 -1 1 (15.18,12) 1 -1 -1 (45.48.51) -1 1 -1 (25,28,19) 1 1 (75.75,81)arrow_forward
- S B Pin 6 mm Garrow_forwardMid-Term Exam 2024/2025 Post graduate/Applied Mechanics- Metallurgy Q1/ State the type of fault in the following case, and state the structure in which it will appear. АВСАВСВАСВАСАВСАВСarrow_forwardالثانية Babakt Momentum equation for Boundary Layer S SS -Txfriction dray Momentum equation for Boundary Layer What laws are important for resolving issues 2 How to draw. 3 What's Point about this.arrow_forward
- R αι g The system given on the left, consists of three pulleys and the depicted vertical ropes. Given: ri J₁, m1 R = 2r; απ r2, J2, m₂ m1; m2; M3 J1 J2 J3 J3, m3 a) Determine the radii 2 and 3.arrow_forwardB: Solid rotating shaft used in the boat with high speed shown in Figure. The amount of power transmitted at the greatest torque is 224 kW with 130 r.p.m. Used DE-Goodman theory to determine the shaft diameter. Take the shaft material is annealed AISI 1030, the endurance limit of 18.86 kpsi and a factor of safety 1. Which criterion is more conservative? Note: all dimensions in mm. 1 AA Motor 300 Thrust Bearing Sprocket 100 9750 เอarrow_forwardQ2: The plate material of a pressure vessel is AISI 1050 QT 205 °C. The plate is rolled to a diameter of 1.2 m. The two sides of the plate are connected via a riveted joint as shown below. If the rivet material is G10500 with HB=197 and all rivet sizes M31. Find the required rivet size when the pressure vessel is subjected to an internal pressure of 500 MPa. Take safety factor = 2. 1.2m A B' A Chope olm 10.5 0.23 hopearrow_forward
 Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
Refrigeration and Air Conditioning Technology (Mi...Mechanical EngineeringISBN:9781305578296Author:John Tomczyk, Eugene Silberstein, Bill Whitman, Bill JohnsonPublisher:Cengage Learning
