
Concept explainers
(a)
Interpretation:
The products of the proton transfer reaction shown below is to be drawn.
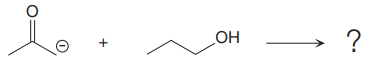
Concept introduction:
In a proton transfer reaction, Bronsted-Lowry base reacts with Bronsted-Lowry acid. Here, Bronsted-Lowry acid donates a proton and forms a conjugate base while Bronsted-Lowry base accepts a proton and forms a conjugate acid. The proton transfer reaction consists of a single elementary step.
(b)
Interpretation:
A free energy diagram for the following reaction, indicating whether it is endothermic or exothermic, is to be drawn.

Concept introduction:
When comparing two acids, the acid with the lower
A reaction tends to be spontaneous if
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- 2. 200 LOD For an unknown compound with a molecular ion of 101 m/z: a. Use the molecular ion to propose at least two molecular formulas. (show your work) b. What is the DU for each of your possible formulas? (show your work) C. Solve the structure and assign each of the following spectra. 8 6 4 2 (ppm) 150 100 50 ō (ppm) 4000 3000 2000 1500 1000 500 HAVENUMBERI-11arrow_forwardComplete the spectroscopy with structurearrow_forwardComplete the spectroscopy with structurearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
