
a.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as
b.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
c.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
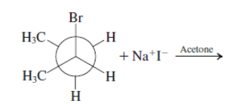
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
d.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
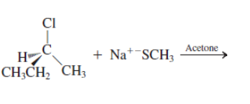
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
e.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
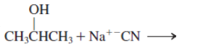
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
f.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
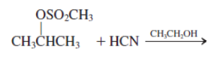
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
g.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
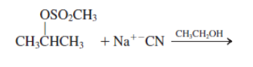
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
h.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
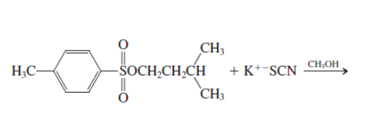
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
i.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
j.
Interpretation: The product for the following reaction needs to be determined if reaction is possible:
Concept introduction: A chemical transformation of one substance (reactants) to the other (products) via single or several steps involved in the reaction is known as chemical reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


