
(a)
Interpretation: The potential organic product that could result from the below reaction should be identified.
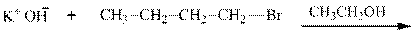
Concept introduction: Bimolecular substitution or
A general
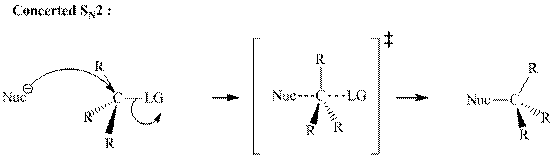
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
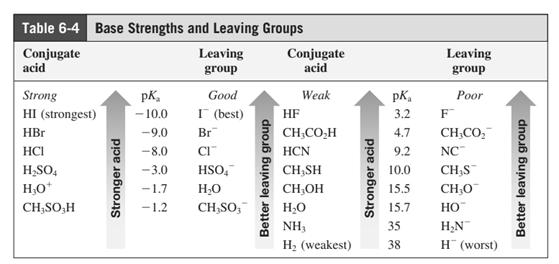
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of the strength of bases is as follows:
(b)
Interpretation:The potential organic product that could result from the below reaction should be identified.
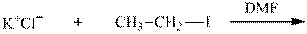
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
In general, the weak conjugate bases that are derived from strong acids are also good leaving groups. The table for leaving groups on the basis of the strength of bases is as follows:
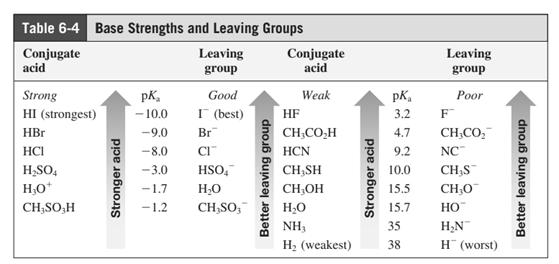
(c)
Interpretation:The potential organic product that could result from the below reaction should be identified.
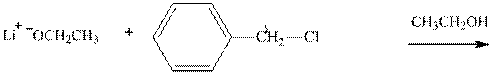
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(d)
Interpretation: The potential organic product that could resultfrom the belowreaction should be identified and no reaction should be indicated if no product is possible.
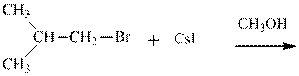
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(e)
Interpretation: The potential organic product that could result from the belowreaction should be identified and no reaction should be indicated if no product is possible.
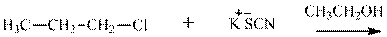
Concept introduction: Bimolecular substitution or
(f)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
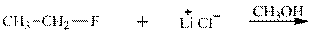
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(g)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
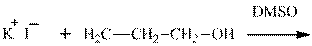
Concept introduction: Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(h)
Interpretation: The potential organic product that could result frombelow reaction should be identified and no reaction should be indicated if no product is possible.
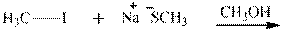
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(i)
Interpretation: The potential organic product that could result from below reaction should be identified and no reaction should be indicated if no product is possible.
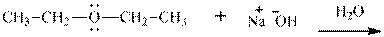
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
(j)
Interpretation: The potential organic product that could result from below reaction should be identified and no reaction should be indicated if no product is possible.
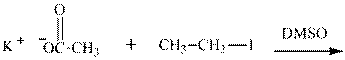
Concept introduction:Bimolecular substitution or
Leaving-group ability is determined by the capacity of leaving the group to accommodate the negative charge as it is displaced from the alkyl halide. Among halogens, the iodides are best-leaving groups followed by bromide chloride and fluoride. Besides halides, some sulphonates and sulphate that can easily delocalize the negative charge can also behave as good leaving group. These include tosylate, mesylate and triflate.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- 19.57 Using one of the reactions in this chapter, give the correct starting material (A-L) needed to produce each structure (a-f). Name the type of reaction used. (b) ہ مرد (d) HO (c) དང་ ་་ཡིན་ད་དང་ (f) HO Br B D of oli H J Br K C 人 ↑arrow_forwardInductive effect (+I and -I) in benzene derivatives.arrow_forward7. Helparrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




