
Concept explainers
(a)
Interpretation: The transformation that synthesizes the product indicated from the below substrate should be proposed.
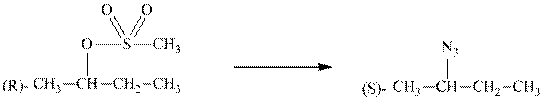
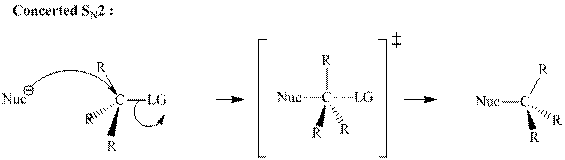
Concept introduction: Bimolecular substitution or
A general
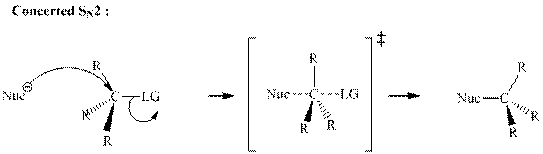
(b)
Interpretation: The transformation that synthesizes the product indicated from the substrate should be proposed.

Concept introduction: Bimolecular substitution or
(c)
Interpretation: The transformation that synthesizes the substituted product indicated with the below substrate should be proposed.
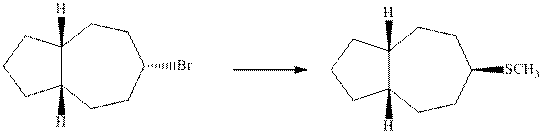
Concept introduction: Bimolecular substitution or
A general
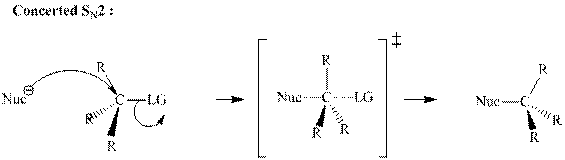
(d)
Interpretation: The transformation that synthesizes the substituted product indicated with the substrate should be proposed.

Concept introduction: Bimolecular substitution or
A general
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
EBK ORGANIC CHEMISTRY
- Nonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forwardPlease help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forward
- Which of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forwardBased on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
