
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 6, Problem 13CTQ
Interpretation Introduction
Interpretation:
The bond-line representation for the following molecule should be predicted.
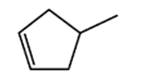
Concept Introduction:
The structure which represents the chains, branches, or rings of bonded atoms in which the atoms are joined together is known as skeletal structure.
The representation of atoms present in a compound without showing the bonds that link them together is known as condensed structural formula.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can u help me figure out the reaction mechanisms for these, idk where to even start
Hi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!
Hi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 6 - Prob. 1CTQCh. 6 - Prob. 2CTQCh. 6 - Prob. 4CTQCh. 6 - Prob. 5CTQCh. 6 - Complete this graph of relative potential energy...Ch. 6 - Prob. 7CTQCh. 6 - Prob. 8CTQCh. 6 - Prob. 9CTQCh. 6 - Consider the Newman projection below. a. Draw a...Ch. 6 - Draw a Newman projection showing the lowest P.E....
Ch. 6 - Prob. 12CTQCh. 6 - Prob. 13CTQCh. 6 - In skeletal representations the hydrogens are not...Ch. 6 - Prob. 15CTQCh. 6 - Prob. 16CTQCh. 6 - Prob. 17CTQCh. 6 - Prob. 19CTQCh. 6 - Prob. 20CTQCh. 6 - Prob. 21CTQCh. 6 - Prob. 22CTQCh. 6 - Prob. 23CTQCh. 6 - Draw a constitutional isomer of pentane,...Ch. 6 - How many H’s are lost from the molecular formula...Ch. 6 - How many ifs are lost from the molecular formula...Ch. 6 - Prob. 27CTQCh. 6 - What is the degree of unsaturation for the example...Ch. 6 - Without counting hydrogens, determine which one of...Ch. 6 - Determine the degree of unsaturation (and draw a...Ch. 6 - a model of each molecule shown above: Is the...Ch. 6 - Prob. 32CTQCh. 6 - Prob. 33CTQCh. 6 - Label each double bond E, Z, or neither. (It may...Ch. 6 - Prob. 35CTQCh. 6 - Prob. 36CTQCh. 6 - Indicate the relationship between each pair....Ch. 6 - Prob. 38CTQCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Using your model of butane (CH3CH2CH2CH3) ,...Ch. 6 - Consider the molecule 1-bromo-2-methylbutane. C3...Ch. 6 - Prob. 5ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - Prob. 13ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Double bonds do not rotate freely under normal...Ch. 6 - up an example (not appearing in this ChemActivity)...Ch. 6 - Prob. 24ECh. 6 - Prob. 25E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forward
- Draw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forwardDraw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forward
- Explain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forwardExplain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forward
- Briefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forwardGiven the hydrazones indicated, draw the structures of the enamines that can be formed. Indicate the most stable enamine (explain). C6H5 C6H5 H C6H5 Harrow_forward4. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning