
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 6CTQ
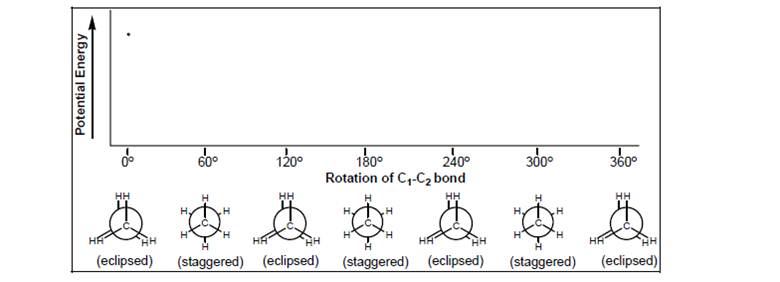
Complete this graph of relative potential energy vs. rotation of the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A solution of 14 g of a nonvolatile, nonelectrolyte compound in 0.10 kg of benzene boils at
81.7°C. If the BP of pure benzene is 80.2°C and the K, of benzene is 2.53°C/m, calculate the
molar mass of the unknown compound. AT₁ = Km (14)
Please help me answer the following questions. My answers weren't good enough. Need to know whyy the following chemicals were not used in this experiment related to the melting points and kf values. For lab notebook not a graded assignments.
Draw the arrow pushing reaction mechanism. DO NOT ANSWER IF YOU WONT DRAW IT. Do not use chat gpt.
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 6 - Prob. 1CTQCh. 6 - Prob. 2CTQCh. 6 - Prob. 4CTQCh. 6 - Prob. 5CTQCh. 6 - Complete this graph of relative potential energy...Ch. 6 - Prob. 7CTQCh. 6 - Prob. 8CTQCh. 6 - Prob. 9CTQCh. 6 - Consider the Newman projection below. a. Draw a...Ch. 6 - Draw a Newman projection showing the lowest P.E....
Ch. 6 - Prob. 12CTQCh. 6 - Prob. 13CTQCh. 6 - In skeletal representations the hydrogens are not...Ch. 6 - Prob. 15CTQCh. 6 - Prob. 16CTQCh. 6 - Prob. 17CTQCh. 6 - Prob. 19CTQCh. 6 - Prob. 20CTQCh. 6 - Prob. 21CTQCh. 6 - Prob. 22CTQCh. 6 - Prob. 23CTQCh. 6 - Draw a constitutional isomer of pentane,...Ch. 6 - How many H’s are lost from the molecular formula...Ch. 6 - How many ifs are lost from the molecular formula...Ch. 6 - Prob. 27CTQCh. 6 - What is the degree of unsaturation for the example...Ch. 6 - Without counting hydrogens, determine which one of...Ch. 6 - Determine the degree of unsaturation (and draw a...Ch. 6 - a model of each molecule shown above: Is the...Ch. 6 - Prob. 32CTQCh. 6 - Prob. 33CTQCh. 6 - Label each double bond E, Z, or neither. (It may...Ch. 6 - Prob. 35CTQCh. 6 - Prob. 36CTQCh. 6 - Indicate the relationship between each pair....Ch. 6 - Prob. 38CTQCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Using your model of butane (CH3CH2CH2CH3) ,...Ch. 6 - Consider the molecule 1-bromo-2-methylbutane. C3...Ch. 6 - Prob. 5ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - Prob. 13ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Double bonds do not rotate freely under normal...Ch. 6 - up an example (not appearing in this ChemActivity)...Ch. 6 - Prob. 24ECh. 6 - Prob. 25E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the following esterification reaction by drawing the structural formula of the product formed. HOH HO i catalyst catalyst OH HO (product has rum flavor) (product has orange flavor)arrow_forwardThe statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard Gibbs free energy of reaction and K stands for the equilibrium constant. In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t Otherwise, check the "no false statements" box under the table. statement false? AG"1 no false statements: statement false? AG-0 0 InK-0 0 K-1 0 AH-TAS no false statements 2arrow_forwardComplete the following esterification reactions by drawing the line formulas of the carboxylic acid and alcohol required to form the ester shown. catalyst catalyst catalyst apricot fragrancearrow_forward
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY