
Concept explainers
Interpretation:
The chemical formula for the carbon tetrachloride should be identified.
Concept introduction:
According to the nomenclature, when two nonmetals are present in the given compound the name of the compound is given as follows, For example, HCl. According to the name of the compound, first give the name for the hydrogen followed by the second element, changing the ending of its name to –ide. Chlorine called as chloride. Therefore HCl is hydrogen chloride. Similarly, HI is hydrogen iodide. SiC is silicon carbide.
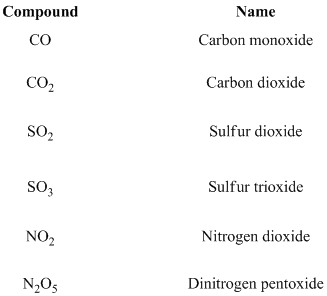
One pair of elements to form several different binary molecular compounds. In thesecases, using of Greek prefixes to denote the number ofatoms of each element present.
Greek prefixes are given below,

If prefix mono substituted is generally omitted for the first element. For example, SO2 is named sulfur dioxide, is not monosulfur dioxide. Moreover, only one atom in a prefix for the first element, no needs to mention mono or di etc.… In addition, for ease of pronunciation, we usually eliminate the last letter of a prefix that ends in o or a when naming an oxide. Thus, N2O5 is dinitrogen pentoxide, rather than dinitrogen pentaoxide.
If any halogens are present in the molecule in suffix, the name of the halogens as follows.

Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Chemistry: Atoms First
- help draw the moleculearrow_forwardHow to draw this claisen condensation reaction mechanisms/arrow_forwardWrite all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forward
- How can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forwarddraw out these molecules pleasearrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning


