
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.4, Problem 13P
Name each of the following:

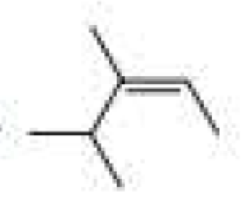
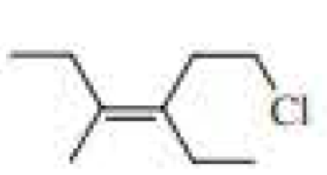
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Done
18:19
www-awu.aleks.com
Chapter 12 HW
Question 27 of 39 (5 points) | Question Attempt: 1 of Unlimited
.. LTE
סוי
9
✓ 20
✓ 21
× 22
23
24
25
26
27
28
29
30
Answer the following questions about the given alkane.
Part: 0 / 2
Part 1 of 2
Classify each carbon atom as a 1º, 2º, 3º, or 4°. Highlight in red any 1°
carbons, highlight in blue any 2° carbons. highlight in green any 3°
carbons, and leave any 4° carbons unhighlighted.
Skip Part
Check
Save For Later
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility
☑
ค
<
Done
19:22
www-awu.aleks.com
Chapter 12 HW
Question 4 of 39 (2 points) | Question Attempt: 5 of Unlimited
:
.. LTE
סוי
1
✓ 2
✓ 3
= 4
✓ 5
✓ 6
✓ 7
✓ 8
✓ 9
= 10
11
✓ 12
Consider the molecule (CH3)2CHCH2CHCн for the following questions.
Part 1 of 2
Which of the following molecules is/are constitutional isomer(s) to
(CH3)2CHCH2CH2CH3? Check all that apply.
Part 2 of 2
(CH3),C(CH2)2CH3
CH3
H,C-CH-CH-CH,
CH 3
None of the above.
☑
Which of the following molecules is/are identical molecules to
(CH3)2CHCH2CH2CH₁₂? Check all that apply.
CH3
H,C-CH-CH₂-CH2-CH,
CH3(CH2)2CH(CH3)2
CH2-CH2-CH3
HỌC-CH=CH,
乂
☑
а
None of the above
Check
Save For Later
Submit Assignment
© 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility
18:11
LTE ا...
US$50 off hotels is waiting for you
Book now, hotels in Nashville are going fast
QUTSLIVII 25 61 69 points) | QuestIVIT ALLēm...
now
Give the IUPAC name for each compound.
Part 1 of 3
Part 2 of 3
X
☑
Х
Check
Save For Later
Submit
© 2025 McGraw Hill LLC. All Rights Reserved.
TOMS CT US
...vacy Center | Accessibility
Chapter 5 Solutions
Organic Chemistry
Ch. 5.1 - Determine the degree of unsaturation and then draw...Ch. 5.1 - Prob. 4PCh. 5.2 - Prob. 5PCh. 5.2 - Draw the structure for each of the following: a....Ch. 5.2 - What is each compounds systematic name?Ch. 5.4 - Assign relative priorities to each set of...Ch. 5.4 - Tamoxifen slows the growth of some breast tumors...Ch. 5.4 - Draw and label the E and Z isomers for each of the...Ch. 5.4 - Prob. 12PCh. 5.4 - Name each of the following:
Ch. 5.4 - Prob. 14PCh. 5.4 - Prob. 15PCh. 5.6 - Prob. 16PCh. 5.6 - Prob. 17PCh. 5.6 - Prob. 18PCh. 5.6 - Prob. 20PCh. 5.7 - a. Which of the monosubstituted cyclohexanes in...Ch. 5.7 - a. for which reaction in each set will S be more...Ch. 5.7 - a. For a reaction with H = 12 kcal/ mol and S =...Ch. 5.7 - Prob. 25PCh. 5.7 - Prob. 26PCh. 5.7 - Prob. 27PCh. 5.9 - The rate constant for a reaction can be increased...Ch. 5.9 - Prob. 30PCh. 5.9 - a. Which reaction has a greater equilibrium...Ch. 5.10 - Draw a reaction coordinate diagram for a two-step...Ch. 5.10 - a. Which step in the reaction coordinate diagram...Ch. 5.10 - Draw a reaction coordinate diagram for the...Ch. 5.11 - Prob. 35PCh. 5 - What is each compounds systematic name?Ch. 5 - Draw the structure of a hydrocarbon that has six...Ch. 5 - Draw the condensed structure for each of the...Ch. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Name the following:Ch. 5 - Prob. 42PCh. 5 - 43. Draw the skeletal structure of...Ch. 5 - In a reaction in which reactant A is in...Ch. 5 - Which bond is stronger? Briefly explain why.Ch. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Assign relative priorities to each set of...Ch. 5 - Prob. 49PCh. 5 - By following the curved red arrows, draw the...Ch. 5 - Prob. 51PCh. 5 - Draw structures for the following: a....Ch. 5 - Prob. 53PCh. 5 - a. Which of the following reactions has the larger...Ch. 5 - a. What is the equilibrium constant for a reaction...Ch. 5 - Prob. 56PCh. 5 - Prob. 57PCh. 5 - Given that the free energy of the twist-boat...Ch. 5 - Prob. 59PCh. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Draw curved arrows to show the movement of the...Ch. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Done 19:17 www-awu.aleks.com Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimited .III LTE סוי 27 28 = 29 30 31 32 = 33 34 35 Consider this structure. CH3CH2CH2 Part 1 of 3 3 CH2 CH2CH3 - C-CH2CH 3 H CH₂ Give the IUPAC name of this structure. 3-ethyl-3,4-dimethylheptane Part: 1/3 Part 2 of 3 Draw the skeletal structure. Skip Part < Check Click and drag to start drawing a structure. Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Хarrow_forward18:57 .III LTE www-awu.aleks.com Chapter 12 HW Question 31 of 39 (8 points) | Question Attem... Give the IUPAC name of each compound. Part 1 of 4 Part 2 of 4 Х Х Check Save For Later Submit © 2025 McGraw Hill LLC. All Rights Reserved. TOMS OF US vacy Center | Accessibilityarrow_forwardWhat is the missing reactant in this organic reaction? CH3-C-CH2-NH2 + R - CH3 O: 0 CH3-N-CH2-C-NH-CH2-C-CH3 + H2O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click anywhere to draw the first atom of your structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forward
- Done 18:17 • www-awu.aleks.com Chapter 12 HW Question 24 of 39 (4 points) | Question Attempt: 1 of Unlimited ▼ 20 ✓ 21 × 22 23 24 25 26 raw the structure corresponding to each IUPAC name. Part 1 of 2 .III LTE 22 27 28 סוי 29 29 3 A skeletal structure corresponding to the IUPAC name 3-ethyl-4-methylhexane. Part 2 of 2 Click and drag to start drawing a structure. A condensed structure corresponding to the IUPAC name 2,2,4- trimethylpentane. Click anywhere to draw the first atom of your structure. Check Save For Later Submit < Х ப: G © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility : Garrow_forwardDone 18:25 www-awu.aleks.com .III LTE Chapter 12 HW Question 29 of 39 (6 points) | Question Attempt: 1 of Unlimi... Oli 23 24 25 26 27 28 29 30 Consider this structure. CH2 CH2CH2 CH2CH2CH₂ C -C. -CH2CH3 H CH Part: 0 / 3 Part 1 of 3 Give the IUPAC name of this structure. Skip Part < Check ☑ Save For Later © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility ....................arrow_forwardCalculate Ecell at 25.0 oC using the following line notation. Zn(s)|Zn+2(aq, 0.900 M)||Cu+2(aq, 0.000200 M)|Cu(s)arrow_forward
- Predict the product of this organic reaction: O OH + H + OH A P + H2O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there isn't any P because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click and drag to start drawing a structure. X G ☐ :arrow_forward0.0994 g of oxalic acid dihydrate is titrated with 10.2 mL of potassium permanganate. Calculate the potassium permanganate concentration. Group of answer choices 0.0433 M 0.135 M 0.0309 M 0.193 Marrow_forwardExperts...can any one help me solve these problems?arrow_forward
- According to standard reduction potential data in Lecture 4-1, which of the following species is the most difficult to reduce? Group of answer choices Zn2+ AgCl(s) Al3+ Ce4+arrow_forwardWhich Group 1 metal reacts with O2(g) to form a metal peroxide (M2O2)? Group of answer choices Li K Rb Naarrow_forwardWhich of the following statements is true regarding the reaction between Group 1 metals and water? Group of answer choices These reactions result in a basic solution. The metals do not actually react easily with water due to the metals' lack of conductivity. These reaction result in an acidic solution. The metals need their outer coatings of metal oxides to react.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY