
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
2nd Edition
ISBN: 9781337086431
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 9RQ
Consider the following mixture of SO2(g) and O2(g).
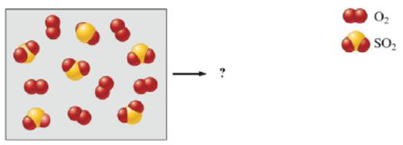
If SO2(g) and O2(g) react to form SO3(g), draw a representation of the product mixture assuming the reaction goes to completion. What is the limiting reactant in the reaction? If 96.0 g of SO2 react with 32.0 g O2, what mass o f product will form?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
10. Complete the substitution reaction of 2 pentanol with these reagents.
Reagents & Reaction Conditions use practice sheet. Please write only
major products, minor product like water, other gases are not
required.
Hint: In substitution of alcohol, we generally substitute OH group
with Halogens like cl, Br, F using some reagent containing
halogens. Ensure to add halogens to the same carbon number
where you are removing OH from
Examples
Alcohols can be converted to Alkyl Halides with HX acids
HBr
H₂O
HCI
+ H₂O
HI
+
H₂O
CH,CH₂OH + SOCI₂
CH,CH₂OH + PCI₁₂
A
BBYJU'S
CH CHCI + SO₂+ HCI
CH₂CH CIP(OH), + HCI
CH,CH₂OH + PCI CHCHCI + POCI + HCI
CH,CH₂OH + PBr, CH,CH,Br + P(OH), + HBr
1. Reaction with HBr with 2 Pentanol
2.Reaction with HI with 2 pentanol
© Byjus.com
3.Reaction with HCI+ZnCl,, with 2 pentanol (Zncl2 is catalyst no role)
4.Reaction with SOCI,, with 2 Pentanol
5.Reaction with PBr; or PCl, with 2 pentanol
3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write
out the
structures of 2-methyl-2-propanol and also any oxidation products of 2-
methyl-2-
propanol. If there is more than one oxidation product, give the structure of
each
of the products.
4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a
common name
by which it is much better known (You'll see it in the grocery store or
pharmacy).
Give that common name
5. Aldehydes can be synthesized by the oxidation of. Please choose from
below choices
A. Primary alcohols
B. Secondary alcohols
C. Organic acids
D. Inorganic acids
6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the
product.
Finish the reactions
hand written please
Chapter 5 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
Ch. 5 - Prob. 1RQCh. 5 - Atomic masses are relative masses. What does this...Ch. 5 - The atomic mass of boron (B) is given in the...Ch. 5 - What three conversion factors and in what order...Ch. 5 - Fig. 5-5 illustrates a schematic diagram of a...Ch. 5 - What is the difference between the empirical and...Ch. 5 - Consider the hypothetical reaction between A2 and...Ch. 5 - Prob. 8RQCh. 5 - Consider the following mixture of SO2(g) and...Ch. 5 - Why is the actual yield of a reaction often less...
Ch. 5 - The following are actual student responses to the...Ch. 5 - What information do we get from a chemical...Ch. 5 - You are making cookies and are missing a key...Ch. 5 - Nitrogen gas (N2) and hydrogen gas (H2) react to...Ch. 5 - For the preceding question, which of the following...Ch. 5 - Prob. 6ALQCh. 5 - Prob. 7ALQCh. 5 - Consider an iron bar on a balance as shown. As the...Ch. 5 - You may have noticed that water sometimes drips...Ch. 5 - Prob. 10ALQCh. 5 - What is true about the chemical properties of the...Ch. 5 - Is there a difference between a homogeneous...Ch. 5 - Prob. 13ALQCh. 5 - The average mass of a carbon atom is 12.011....Ch. 5 - Can the subscripts in a chemical formula be...Ch. 5 - Consider the equation 2A + B . A2B. If you mix 1.0...Ch. 5 - According to the law of conservation of mass, mass...Ch. 5 - Which of the following pairs of compounds have the...Ch. 5 - Atoms of three different elements are represented...Ch. 5 - In chemistry, what is meant by the term mole? What...Ch. 5 - Which (if any) of the following is (are) true...Ch. 5 - Consider the equation 3A + B C + D. You react 4...Ch. 5 - Reference Section 5-2 to find the atomic masses of...Ch. 5 - Avogadros number, molar mass, and the chemical...Ch. 5 - If you had a mole of U.S. dollar bills and equally...Ch. 5 - Prob. 26QCh. 5 - Which of the following compounds have the same...Ch. 5 - Prob. 28QCh. 5 - How is the mass percent of elements in a compound...Ch. 5 - A balanced chemical equation contains a large...Ch. 5 - Prob. 31QCh. 5 - Hydrogen gas and oxygen gas react to form water,...Ch. 5 - What is the theoretical yield for a reaction, and...Ch. 5 - What does it mean to say a reactant is present in...Ch. 5 - Consider the following generic reaction: A2B2 + 2C...Ch. 5 - Consider the following generic reaction:...Ch. 5 - An element consists of 1.40% of an isotope with...Ch. 5 - An element X bas five major isotopes, which are...Ch. 5 - The element rhenium (Re) bas two naturally...Ch. 5 - Assume silicon has three major isotopes in nature...Ch. 5 - The element europium exists in nature as two...Ch. 5 - The element silver (Ag) has two naturally...Ch. 5 - The mass spectrum of bromine (Br2) consists of...Ch. 5 - The stable isotopes of iron arc 54Fe, 56Fe, 57Fe,...Ch. 5 - Calculate the mass of 500. atoms of iron (Fe).Ch. 5 - What number of Fe atoms and what amount (moles) of...Ch. 5 - Diamond is a natural form of pure carbon. What...Ch. 5 - A diamond contains 5.0 1021 atoms of carbon. What...Ch. 5 - Aluminum metal is produced by passing an electric...Ch. 5 - The Freons are a class of compounds containing...Ch. 5 - Calculate the molar mass of the following...Ch. 5 - Calculate the molar mass of the following...Ch. 5 - What amount (moles) of compound is present in 1.00...Ch. 5 - What amount (moles) of compound is present in 1.00...Ch. 5 - What mass of compound is present in 5.00 moles of...Ch. 5 - What mass of compound is present in 5.00 moles of...Ch. 5 - Prob. 57ECh. 5 - Prob. 58ECh. 5 - Prob. 59ECh. 5 - What number of molecules (or formula units) are...Ch. 5 - What number of atoms of nitrogen are present in...Ch. 5 - Prob. 62ECh. 5 - Freon- 12 (CCI2F2) is used as a refrigerant in air...Ch. 5 - Bauxite, the principal ore used in the production...Ch. 5 - What amount (moles) is represented by each of...Ch. 5 - What amount (moles) is represented by each of...Ch. 5 - What number of atoms of nitrogen are present in...Ch. 5 - Complete the following table.Ch. 5 - Ascorbic acid, or vitamin C (C6H8O6), is an...Ch. 5 - The molecular formula of acetylsalicylic acid...Ch. 5 - Chloral hydrate (C2H3Cl3O2) is a drug formerly...Ch. 5 - Dimethylnitrosamine, (CH3)2N2O , is a carcinogenic...Ch. 5 - Calculate the percent composition by mass of the...Ch. 5 - In 1987 the first substance to act as a...Ch. 5 - Prob. 75ECh. 5 - Arrange the following substances in order of...Ch. 5 - Fungal laccase, a blue protein found in...Ch. 5 - Hemoglobin is the protein that transports oxygen...Ch. 5 - Express the composition of each of the following...Ch. 5 - Considering your answer to Exercise 79, which type...Ch. 5 - Give the empirical formula for each of the...Ch. 5 - Determine the molecular formulas to which the...Ch. 5 - A compound that contains only carbon, hydrogen,...Ch. 5 - The most common form of nylon (nylon-6) is 63.68%...Ch. 5 - There are two binary compounds of mercury and...Ch. 5 - A sample of urea contains 1.121 g N, 0.161 g H,...Ch. 5 - Prob. 87ECh. 5 - Determine the molecular formula of a compound that...Ch. 5 - A compound contains 47.08% carbon, 6.59% hydrogen,...Ch. 5 - Maleic acid is an organic compound composed of...Ch. 5 - One of the components that make up common table...Ch. 5 - A compound contains only C, H, and N. Combustion...Ch. 5 - Prob. 93ECh. 5 - A compound contains only carbon, hydrogen, and...Ch. 5 - Give the balanced equation for each of the...Ch. 5 - Give the balanced equation for each of the...Ch. 5 - Prob. 97ECh. 5 - Iron oxide ores, commonly a mixture of FeO and...Ch. 5 - Balance the following equations: a. Ca(OH)2(aq) +...Ch. 5 - Balance each of the following chemical equations....Ch. 5 - Prob. 101ECh. 5 - Balance the following equations: a. Cr(s) + S8(s) ...Ch. 5 - Silicon is produced for the chemical and...Ch. 5 - Prob. 104ECh. 5 - Over the years, the thermite reaction has been...Ch. 5 - The reaction between potassium chlorate and red...Ch. 5 - The reusable booster rockets of the U.S. space...Ch. 5 - One of relatively few reactions that takes place...Ch. 5 - Elixirs such as Atka-Seltzer use the reaction of...Ch. 5 - Aspirin (C9H8O4) is synthesized by reacting...Ch. 5 - Bacterial digestion is an economical method of...Ch. 5 - Phosphorus can be prepared from calcium phosphate...Ch. 5 - Coke is an impure form of carbon that is often...Ch. 5 - The space shuttle environmental control system...Ch. 5 - Consider the reaction between NO(g) and O2(g)...Ch. 5 - Consider the following reaction:...Ch. 5 - Prob. 117ECh. 5 - Consider the following unbalanced equation:...Ch. 5 - Hydrogen peroxide is used as a cleansing agent in...Ch. 5 - Silver sulfadiazine bum-treating cream creates a...Ch. 5 - Hydrogen cyanide is produced industrially from the...Ch. 5 - Acrylonitrile C3H3N) is the starting material for...Ch. 5 - Prob. 123ECh. 5 - DDT, an insecticide harmful to fish, birds, and...Ch. 5 - Bornite (Cu3FeS3) is a copper ore used in the...Ch. 5 - Consider the following unbalanced reaction:...Ch. 5 - In using a mass spectrometer, a chemist sees a...Ch. 5 - Boron consists of two isotopes, 10B and 11B....Ch. 5 - A given sample of a xenon fluoride compound...Ch. 5 - Aspartame is an artificial sweetener that is 160...Ch. 5 - Anabolic steroids are performance enhancement...Ch. 5 - Many cereals are made with high moisture content...Ch. 5 - The compound adrenaline contains 56.79% C, 6.56%...Ch. 5 - Adipic acid is an organic compound composed of...Ch. 5 - Prob. 135AECh. 5 - Some bismuth tablets, a medication used to treat...Ch. 5 - The empirical formula of styrene is CH; the molar...Ch. 5 - Terephthalic acid is an important chemical used in...Ch. 5 - A sample of a hydrocarbon (a compound consisting...Ch. 5 - A binary compound between an unknown element E and...Ch. 5 - A 0.755-g sample of hydrated copper(II) sulfate...Ch. 5 - ABS plastic is a tough, hard plastic used in...Ch. 5 - Prob. 143AECh. 5 - Methane (CH4) is the main component of marsh gas....Ch. 5 - A potential fuel for rockets is a combination of...Ch. 5 - A 0.4230-g sample of impure sodium nitrate was...Ch. 5 - Prob. 147AECh. 5 - Commercial brass, an alloy of Zn and Cu, reacts...Ch. 5 - Prob. 149AECh. 5 - You have seven closed containers, each with equal...Ch. 5 - A substance X2Z has the composition (by mass) of...Ch. 5 - Consider samples of phosphine (PH3), water (H2O),...Ch. 5 - Calculate the number of moles for each compound in...Ch. 5 - Arrange the following substances in order of...Ch. 5 - Para-cresol, a substance used as a disinfectant...Ch. 5 - A compound with molar mass 180.1 g/mol has the...Ch. 5 - Prob. 157CWPCh. 5 - Consider the following unbalanced chemical...Ch. 5 - Sulfur dioxide gas reacts with sodium hydroxide to...Ch. 5 - Gallium arsenide, GaAs, has gained widespread use...Ch. 5 - Consider the following data for three binary...Ch. 5 - Natural rubidium has the average mass of 85.4678 u...Ch. 5 - A compound contains only carbon, hydrogen,...Ch. 5 - Nitric acid is produced commercially by the...Ch. 5 - When the supply of oxygen is limited, iron metal...Ch. 5 - A 9.780-g gaseous mixture contains ethane (C2H6)...Ch. 5 - Zinc and magnesium metal each reacts with...Ch. 5 - Prob. 168CPCh. 5 - Consider a gaseous binary compound with a molar...Ch. 5 - Prob. 170CPCh. 5 - Prob. 171CPCh. 5 - The aspirin substitute, acetaminophen (C8H9O2N),...Ch. 5 - An element X forms both a dichloride (XCl2) and a...Ch. 5 - Prob. 174CPCh. 5 - When aluminum metal is heated with an element from...Ch. 5 - Consider a mixture of potassium chloride and...Ch. 5 - Ammonia reacts with O2 to form either NO(g) or...Ch. 5 - You take 1.00 g of an aspirin tablet (a compound...Ch. 5 - With the advent of techniques such as scanning...Ch. 5 - Tetrodotoxin is a toxic chemical found in fugu...Ch. 5 - Prob. 181IPCh. 5 - Prob. 182IPCh. 5 - Prob. 183IPCh. 5 - A 2.077-g sample of an element, which has an...Ch. 5 - Consider the following balanced chemical equation:...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Identify each alcohol as primary, secondary, or tertiary Drag the appropriate items to their respective bins. CH₂ H₂C- -C-OH HO CH₂ Primary Он OH CH₂ OH CCH₂OH CH₂ сн Secondary Tertiary Reset Help CH,CH₂ (CH)CHCH,OH CH,CH,CH,CCH, CHOH CH₂ Different types of alcohol groups Alcohol and its reaction: 8. Combing two alcohol molecules below and completing the reaction with Product .( Hint Reaction called etherification as ether is formed and name the ether once you complete the reaction. Hint.: R-O-H+H-O-RR-O-R Do the reaction: CH₂OH + CH₂OH---→ + H-O-H 9. Write the reaction of formation of alcohol from alkene by adding water: Addition reaction also called hydration reaction as we are adding water which occur always in presence of acid Hint: Break the double bond and add H and OH if symmetrical then add anywhere if unsymmetrical then follow Markovnikov rule H should go to that double bone carbon which has more hydrogen CH2=CH2 + H₂O-→arrow_forwardComplete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forward
- b) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forwardIndicate the reaction products when CH3COCH2COOCH2COOC2H5 (ethyl acetoacetoacetate) reacts with 1º OH-/H2O and 2º H3O+arrow_forwardDraw the formula of the compound 4-cyclohexyl butanamide?arrow_forward
- What is the formula of the compound 3-isopropylcyclopentane-1-carbonyl chloride?arrow_forwardIndicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forward
- Indicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forwardPrimary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY