
Concept explainers
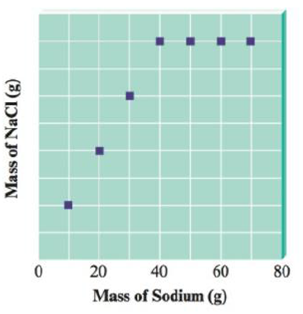
You have seven closed containers, each with equal masses of chlorine gas (Cl2) . You add 10.0 g of sodium to the first sample, 20.0 g of sodium to the second sample, and so on (adding 70.0 g of sodium to the seventh sample). Sodium and chlorine react to form sodium chloride according to the equation
After each reaction is complete, you collect and measure the amount of sodium chloride formed. A graph of your results is shown below.
Answer the following questions:
a. Explain the shape of the graph.
b. Calculate the mass of NaCl formed when 20.0 g of sodium is used.
c. Calculate the mass of Cl2 in each container.
d. Calculate the mass of NaCl formed when 50.0 g of sodium is used.
e. Identify the leftover reactant, and determine its mass for parts b and d above.
(a)
Interpretation: Seven closed containers, having equal amount of chlorine gas present in them, have been given. Sodium is added to the containers in the given pattern. After the completion of the reaction between sodium and chlorine, the amount of sodium chloride formed is measured. The stated questions related to the given process are to be answered.
Concept introduction: The amount of the sodium chloride formed depends upon the amount of sodium and chlorine gas present in each of the given containers.
To determine: The explanation regarding the shape of the graph.
Explanation of Solution
The amount of sodium chloride formed increases on increasing the quantity of sodium from the container one to four.
In the fifth container the addition of a greater amount of sodium than added to the previous container did not increase the amount of sodium chloride formed.
This indicates that the amount of chlorine gas present in the respective container is not sufficient to react with amount of sodium been added to the container. Hence, the amount of sodium chloride formed in the subsequent containers has a constant value.
The amount of chlorine gas present in the containers five, six and seven reacts with an equal amount of sodium to form an equal amount of sodium chloride.
(b)
Interpretation: Seven closed containers, having equal amount of chlorine gas present in them, have been given. Sodium is added to the containers in the given pattern. After the completion of the reaction between sodium and chlorine, the amount of sodium chloride formed is measured. The stated questions related to the given process are to be answered.
Concept introduction: The amount of the sodium chloride formed depends upon the amount of sodium and chlorine gas present in each of the given containers.
To determine: The mass of
Explanation of Solution
Given
The stated reaction is,
The atomic mass of sodium is
The molar mass of
According to the given reaction,
The mass of
(c)
Interpretation: Seven closed containers, having equal amount of chlorine gas present in them, have been given. Sodium is added to the containers in the given pattern. After the completion of the reaction between sodium and chlorine, the amount of sodium chloride formed is measured. The stated questions related to the given process are to be answered.
Concept introduction: The amount of the sodium chloride formed depends upon the amount of sodium and chlorine gas present in each of the given containers.
To determine: The mass of
Explanation of Solution
Given
The stated reaction is,
The atomic mass of sodium is
The molar mass of
According to the given graph, chlorine gas present reacts completely with
According to the given reaction,
The mass of
(d)
Interpretation: Seven closed containers, having equal amount of chlorine gas present in them, have been given. Sodium is added to the containers in the given pattern. After the completion of the reaction between sodium and chlorine, the amount of sodium chloride formed is measured. The stated questions related to the given process are to be answered.
Concept introduction: The amount of the sodium chloride formed depends upon the amount of sodium and chlorine gas present in each of the given containers.
To determine: The mass of
Explanation of Solution
Given
The stated reaction is,
The atomic mass of sodium is
The molar mass of
According to the given graph, chlorine gas present reacts completely with
Hence, only
According to the given reaction,
The mass of
(e)
Interpretation: Seven closed containers, having equal amount of chlorine gas present in them, have been given. Sodium is added to the containers in the given pattern. After the completion of the reaction between sodium and chlorine, the amount of sodium chloride formed is measured. The stated questions related to the given process are to be answered.
Concept introduction: The amount of the sodium chloride formed depends upon the amount of sodium and chlorine gas present in each of the given containers.
To determine: The left over reactant in parts (b) and (d) and their respective mass.
Explanation of Solution
The leftover reactant in part (b) is the chlorine gas. The mass of chlorine left unreacted is
To determine: The left over reactant in parts (b) and its respective mass.
The leftover reactant in part (b) is the chlorine gas. The mass of chlorine left unreacted is
Given
The stated reaction is,
The atomic mass of sodium is
The molar mass of
According to the given reaction,
The mass of chlorine present in the container is
The amount of chlorine left unreacted is
To determine: The left over reactant in parts (d) and its respective mass.
The leftover reactant in part (d) is sodium. The mass of sodium left unreacted is
According to the given graph, chlorine gas present reacts completely with
Hence, only
The amount of sodium left unreacted is
The mass of chlorine gas left unreacted in the stated reaction
The mass of sodium left unreacted in the stated reaction
Want to see more full solutions like this?
Chapter 5 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
- ME EX1) Prblm #19-20 I'm so confused with these problems. Can you please help me solve them and explain them? Problems number 19-20, and thanks! step by step and in detail for me please helparrow_forwardCalculate the flux of oxygen between the ocean and the atmosphere, given that: Temp = 18°C Salinity = 35 ppt Density = 1025 kg/m3 Oxygen concentration measured in bulk water = 263.84 mmol/m3 Wind speed = 7.4 m/s Oxygen is observed to be about 10% initially supersaturatedarrow_forward( ME EX1) Prblm 27-28: Can you explain to me both prblms in detail and for prblm 28 what do you mean bi conjugated bi ponds and those structures I'm confused...arrow_forward
- A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation). B. Specify whether the species is "a"-aromatic, "aa"-anti-aromatic, or "na"-non-aromatic (neither aromatic nor anti-aromatic). (Presume rings to be planar unless structure obviously prevents planarity. If there is more than one conjugated ring, count electrons in the largest.) 1. A.Electrons in a cyclic conjugated system. 18 B.The compound is (a, aa, or na) a 2. A.Electrons in a cyclic conjugated system. 10 B.The compound is (a, aa, or na) naarrow_forwardWater is boiling at 1 atm pressure in a stainless steel pan on an electric range. It is observed that 2 kg of liquid water evaporates in 30 min. Find the rate of heat transfer to the water (kW).arrow_forwardCould you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the resonance structures that were given please.arrow_forward
- Could you please turn this into a complete Lewis dot structure formula for me so I can visualize it more clearly? and then do the explaining for the question.arrow_forwardplease solve. If the answer is "no error" and it asks me to type something, and i typed a-helix, its always wrong.arrow_forwardCan you please solve and explain this for me in a simple way? I cant seem to comprehend this problem.arrow_forward
- Part I. Problem solving. Include all necessary calculations 13 provide plots and graphs. Complexation wl diphenyl carbazide (OPC) in acidic media is another type of sensitive photometric method used for the analysis of aqueous. hexavalent chromium. At 540nm the cherry-red complex as a result of DPC reaction w/ chromium can be photometrically measured. at this wavelength. - a 25mL The UV-vis analysis for the determination of nexavalent chromium in ground water sample is given below. The experiment was based on external calibration method w/ each measurement sample prepared are as follows lab sample analysis contained the standard 100 ppb croy cor groundwater sample, volumes used as indicated below), 12.50 mL of 0.02 M H2Soy and 5.50 ml of 100 ppm DPC (wi water to adjust final volume to 25-ml). The main stripping method was square wave voltammetry, following the conditions set in the main ASV experiment. Standard 100 Volumetric Groundwater H2SO4 0.20 M, flask Sample, mL ppb CrO4*, 100…arrow_forwardplease helparrow_forwardPredict the products of the following reactions. Draw mechanism arrows for each step for a, b, and c. a.) HBr b.) HI H₂O H2SO4 d.) C12 HO H2SO4 1.) BH3 2.) H2O2, NaOHarrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





