
Concept explainers
5-114 Carbon dioxide gas, saturated with water vapor, can be produced by the addition of aqueous acid to calcium carbonate based on the following balanced net ionic equation:
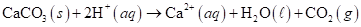
(a) How many moles of wet CO (g), collected at 60.°C and 774 torr total pressure, are produced by the complete reaction of 10.0 g of CaCO3 with excess acid?
(b) What volume does this wet CO2 occupy?
(c) What volume would the CO2 occupy at 774 torr if a desiccant (a chemical drying agent) were added to remove the water? The vapor pressure of water at 60.°C is 149.4 mm Hg.
(a)
Interpretation:
The number of moles of wet
Concept Introduction:
Moles of wet
Answer to Problem 86P
The number of moles of carbon dioxide produced is
Explanation of Solution
The weight of calcium carbonate is
The temperature is
The pressure is
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation,
Therefore, the number of moles of carbon dioxide produced is
(b)
Interpretation:
The volume of wet
Concept Introduction:
To determine the volume of wet
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation.
Answer to Problem 86P
The value of carbon dioxide produced is
Explanation of Solution
The weight of calcium carbonate is
The temperature is.
The pressure is
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation:
Therefore, the number of moles of carbon dioxide produced is
We know that at STP, 1 mole of any gas occupies
Therefore,
Therefore, the volume of
At STP, the value of
Now, convert the pressure from
We know that,
Therefore, the pressure is calculated below:
Now, convert temperature from
We know that,
Therefore,
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation:
Upon rearranging, we get,
By substituting the values of
Hence the value of carbon dioxide produced is
(c)
Interpretation:
The volume of wet
Concept Introduction:
To determine the volume of wet
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation.
Answer to Problem 86P
Explanation of Solution
The weight of calcium carbonate is
The temperature is
The pressure is
The vapor pressure of water at
The molecular weight of calcium carbonate is calculated below:
The number of moles in
The balanced chemical equation is below:
From the above equation:
Therefore, the number of moles of carbon dioxide produced is
We know that at STP, 1 mole of any gas occupies
Therefore,
Therefore, the volume of
At STP, the value of
The pressure is
Now, convert the pressure from
We know that,
Therefore, the pressure is calculated below,
The vapor pressure of water at
Therefore, the pressure is calculated below,
Now, convert temperature from°C to K.
We know that,
Therefore,
The relationship between pressure, volume, and temperature, under two sets of conditions, is given by following equation,
Upon rearranging, we get,
By substituting the values of
Hence the value of carbon dioxide produced is
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction To General, Organic, And Biochemistry
- Can you help me and explain the answers please.arrow_forwardB 1 of 2 Additional problems in preparation to Midterm #1: 1.) How can the following compounds be prepared using Diels-Alder reaction: CH3 O CN (a) (b) CN CH3 2.) What is the missing reagent in the shown reaction? H3C + ? H3C H3C CN H3C ''CN (၁) H 3.) Write the products 1,2-addition and 1,4-addition of DBr to 1,3-cyclohexadiene. Remember, D is deuterium, a heavy isotope of hydrogen. It reacts exactly like hydrogen. 4.) In the shown reaction, which will be the kinetic product and which will be the thermodynamic product? H3C CI H3C HCI H3C + 5.) Which of the following molecules is aromatic? (a) (b) (c) H 6.) Which of the following molecules is aromatic? (a) (b) (c) 7.) Write the mechanism for the shown reaction. + Ха AICI 3 CI 8.) Suggest reagents that would convert benzene into the shown compounds. CI NO2 -8-6-6-8-a (a) (b) (c) (d) (e) (a) SO3H Brarrow_forwardThe number of 2sp^2 hybridized atoms in is: A. 8; B. 6; C.4; D.2; E.0;arrow_forward
- The highest boiling compound from among the following isA. 2-methylheptane; B. 3-methylheptane; C. 2,2-dimethylhexane;D. octane; E. 2,2,3-trimethylpentanearrow_forwardWhich of the following features are found in the most stable structure ofCH5NO that does not have a CO bond?w. a π bond, x. two NH bonds, y. one OH bond, z. 3 lone pairsA. w, x; B. x, y; C. y, z; D. x, y, z; E. all of them.arrow_forwardWhich one of the following functional groups is not present in thecompound shownA. amine; B. aldehyde, C. ether; D. amide. E. ketonearrow_forward
- Which of the following formulas correspond to at least one compound inwhich resonance is important?w. C2H5N x. C3H5Br; y. C3H4; z. C4H6.A. w, x, y; B. x, y, z; C. w, x, z; D. w, y, z; E. all of themarrow_forwardPredict the product(s) that are formed after each step for reactions 1-4. In each case, consider formation of any chiral center(s) and draw all expected stereoisomers. 1) OH 1) HBr (SN2) 2) NaOH, heat 3) BH3, THF 4) H2O2, NaOH 2) OH 1) SOCI 2, py 2) NaOEt 3) Br2, H₂O 3) OH 1) H2SO4 conc. 2) HBr, ROOR 3) KOtBu 4) OH 1) TsCl, py 2) NaOEt 3) 03 4) DMSarrow_forwardWhich of the following rings has the least strain in its most stableconformation?A. Cyclobutane; B. Cyclopentane; C. Cyclohexane; D. Cycloheptane;E. Cyclooctanearrow_forward
- The number of different carbon skeletons that have a main chain of 9carbons and an ethyl branch isA 3; B. 4; C. 5; D. 6; E. 7arrow_forwardQ5: Classify the following pair of molecules as constitutional isomers, enantiomers, diastereomers, the same molecule, or completely different molecules. Br O CI Br OH OH 111 Br .!!!/Br F OH and ...m Br Br OH CI Br OH ་་་་་" ། ་arrow_forwardConsidering only rotation around the 1-2 bond, how many differentstaggered conformations are there of 1,2-dibromo-1,2-dichloropropane?A: 2; B. 3; C. 4; D. 6; E. more than 6.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




