
Concept explainers
Interpretation:
The partial molar volume of
Concept Introduction:
The partial molar volume is the contribution that a component of mixture makes to the total volume of a sample. The partial molar volume of a substance is expressed as,
Answer to Problem 5A.5P
The partial molar volume of
| 5 | 1.0151 | 0.006 | -15.946 |
| 10 | 1.107 | 0.012 | -10.357 |
| 15 | 1.167 | 0.0195 | -3.37075 |
| 20 | 1.23 | 0.027 | 3.6155 |
The graph between mole fraction of
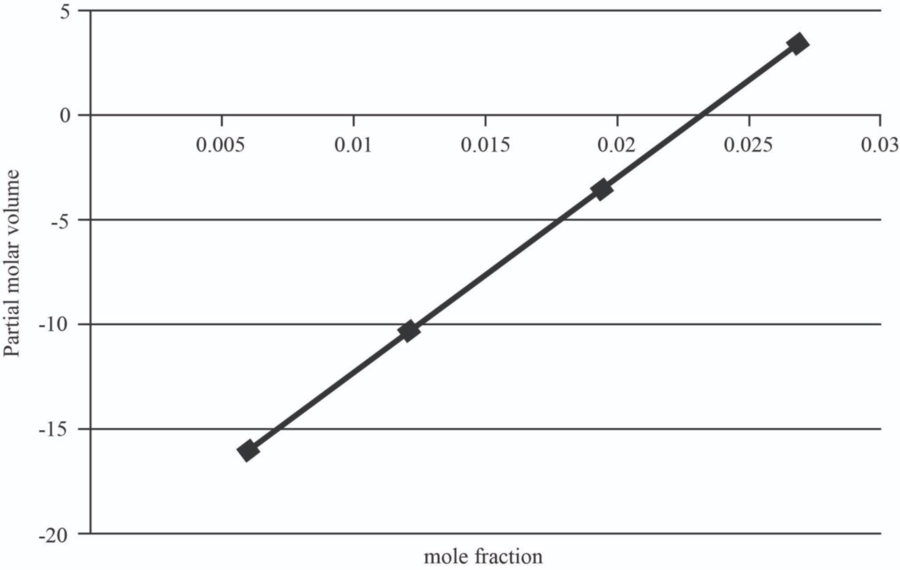
Figure 2
Explanation of Solution
The data of mass densities of aqueous solution of
| 5 | 1.0151 |
| 10 | 1.107 |
| 15 | 1.167 |
| 20 | 1.23 |
The moles of
The molar mass of
Substitute the molar mass of
The density of the solution (
Where,
Therefore, the molar density of the solution (
Where,
The volume of the solution is given by the formula below.
The mass of the solution is
Substitute the value of mass of solution in (3).
Substitute the value of
The mass of water (
Substitute the value of
The total molar volume of the solution (
Substitute the value of
The mass of
The density (
The molar mass of
The molar mass of
Substitute the value of
The mole fraction of
The mass of
Substitute the mass of
Similarly the values of
| 5 | 1.0151 | 0.006 | 17.94 |
| 10 | 1.107 | 0.012 | 17.86 |
| 15 | 1.167 | 0.0195 | 17.81 |
| 20 | 1.23 | 0.027 | 17.81 |
The values of
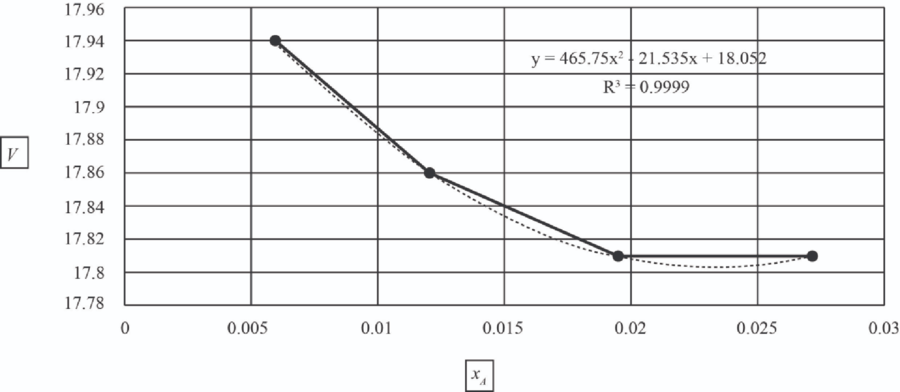
Figure 1
The data was fit to a second order polynomial using the POLY program to generate the equation given below.
The partial molar volume of a substance (
Where,
Differentiate equation (9) with respect to
Substitute the value of
Similarly the values of
| 0.006 | -15.946 |
| 0.012 | -10.357 |
| 0.0195 | -3.37075 |
| 0.027 | 3.6155 |
The graph between mole fraction of
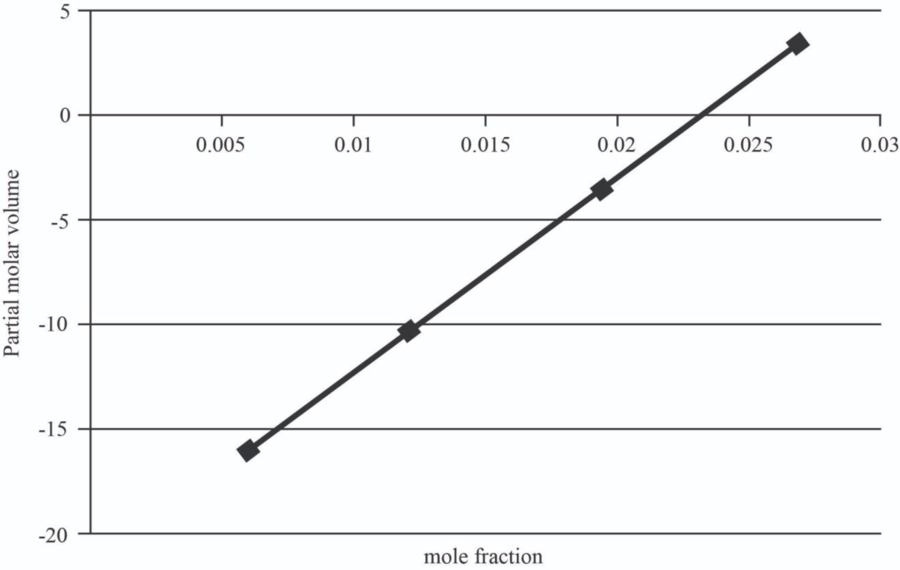
Figure 2
Want to see more full solutions like this?
Chapter 5 Solutions
PHYSICAL CHEMISTRY. VOL.1+2 (LL)(11TH)
- CH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward2.0arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forwardA certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward
- 2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forwardCommercial bleach contains either chlorine or oxygen as an active ingredient. A commercial oxygenated bleach is much safer to handle and less likely to ruin your clothes. It is possible to determine the amount of active ingredient in an oxygenated bleach product by performing a redox titration. The balance reaction for such a titration is: 6H+ +5H2O2 +2MnO4- à 5O2 + 2Mn2+ + 8H2O If you performed the following procedure: “First, dilute the Seventh Generation Non-Chlorine Bleach by pipetting 10 mL of bleach in a 100 mL volumetric flask and filling the flask to the mark with distilled water. Next, pipet 10 mL of the diluted bleach solution into a 250 mL Erlenmeyer flask and add 20 mL of 1.0 M H2SO4 to the flask. This solution should be titrated with 0.0100 M KMnO4 solution.” It took 18.47mL of the KMnO4 to reach the endpoint on average. What was the concentration of H2O2 in the original bleach solution in weight % assuming the density of bleach is 1g/mL?arrow_forward
- 10.arrow_forwardProper care of pH electrodes: Why can you not store a pH electrode in distilled water? What must you instead store it in? Why?arrow_forwardWrite the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 569 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! §arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





