
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.55QE
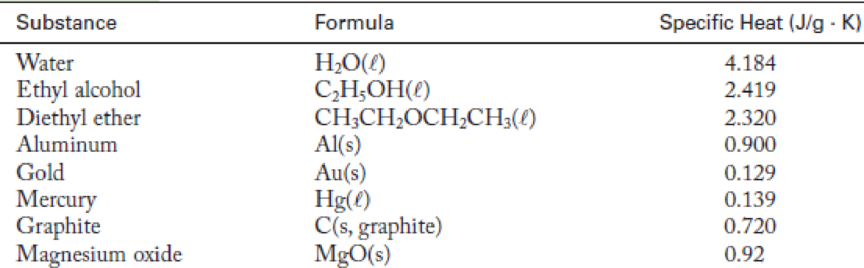
How much heat, in kilojoules, must be removed to decrease the temperature of a 20.0-g bar of aluminum from 34.2 °C to 22.5 °C? (See Table 5.1 for the specific heat of aluminum.)
TABLE 5.1 Specific Heats of Some Common Substances
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Construct a molecular orbital diagram for carbon monoxide. Identify the relevant point group,include all of the appropriate symmetry labels and pictures, and fill in the electrons. Make sure toaccount for the difference in electronegativity between C and O. Hint: CO is substantiallyisoelectronic to N2. (PLEASE DRAW THE ENTIRE MO DIAGRAM!!!)
please help with hw
help me solve this hw
Chapter 5 Solutions
Chemistry: Principles and Practice
Ch. 5 - Why must the physical states of all reactants and...Ch. 5 - Why is chemical energy classified as a form of...Ch. 5 - What is the difference between the enthalpy of...Ch. 5 - Classify each process as exothermic or...Ch. 5 - Explain why the specific heat of the contents of...Ch. 5 - Prob. 5.6QECh. 5 - Define heat. What are its units? How does it...Ch. 5 - Prob. 5.8QECh. 5 - Prob. 5.9QECh. 5 - Prob. 5.10QE
Ch. 5 - Prob. 5.11QECh. 5 - Is the Sun exothermic or endothermic? Is it any...Ch. 5 - Under what circumstances is the heat of a process...Ch. 5 - Prob. 5.14QECh. 5 - Prob. 5.15QECh. 5 - Prob. 5.16QECh. 5 - Prob. 5.17QECh. 5 - Prob. 5.18QECh. 5 - Prob. 5.19QECh. 5 - Prob. 5.20QECh. 5 - Prob. 5.21QECh. 5 - Prob. 5.22QECh. 5 - Prob. 5.23QECh. 5 - Prob. 5.24QECh. 5 - Prob. 5.25QECh. 5 - Prob. 5.26QECh. 5 - Prob. 5.27QECh. 5 - Prob. 5.28QECh. 5 - Prob. 5.29QECh. 5 - Prob. 5.30QECh. 5 - Prob. 5.31QECh. 5 - A chemical reaction occurs and absorbs 64.7 cal....Ch. 5 - The enthalpy change for the following reaction is...Ch. 5 - Prob. 5.34QECh. 5 - The thermochemical equation for the burning of...Ch. 5 - When lightning strikes, the energy can force...Ch. 5 - One step in the manufacturing of sulfuric acid is...Ch. 5 - If nitric acid were sufficiently heated, it can be...Ch. 5 - Prob. 5.39QECh. 5 - Prob. 5.40QECh. 5 - Prob. 5.41QECh. 5 - The combustion of 1.00 mol liquid methyl alcohol...Ch. 5 - Another reaction that is used to propel rockets is...Ch. 5 - Prob. 5.44QECh. 5 - Prob. 5.45QECh. 5 - Prob. 5.46QECh. 5 - Prob. 5.47QECh. 5 - Prob. 5.48QECh. 5 - Prob. 5.49QECh. 5 - Prob. 5.50QECh. 5 - The enthalpy change when 1 mol methane (CH4) is...Ch. 5 - Prob. 5.52QECh. 5 - Prob. 5.53QECh. 5 - How much energy is required to raise the...Ch. 5 - How much heat, in kilojoules, must be removed to...Ch. 5 - Prob. 5.56QECh. 5 - Prob. 5.57QECh. 5 - Prob. 5.58QECh. 5 - Prob. 5.59QECh. 5 - Prob. 5.60QECh. 5 - When 7.11 g NH4NO3 is added to 100 mL water, the...Ch. 5 - A 50-mL solution of a dilute AgNO3 solution is...Ch. 5 - A 0.470-g sample of magnesium reacts with 200 g...Ch. 5 - Dissolving 6.00 g CaCl2 in 300 mL of water causes...Ch. 5 - Draw an energy-level diagram (e.g., see Figure...Ch. 5 - Prob. 5.66QECh. 5 - Prob. 5.67QECh. 5 - Prob. 5.68QECh. 5 - Calculate H for the reaction...Ch. 5 - Prob. 5.70QECh. 5 - Given the thermochemical equations...Ch. 5 - In the process of isolating iron from its ores,...Ch. 5 - Prob. 5.73QECh. 5 - Prob. 5.75QECh. 5 - Prob. 5.77QECh. 5 - Prob. 5.78QECh. 5 - Prob. 5.79QECh. 5 - Prob. 5.80QECh. 5 - Prob. 5.81QECh. 5 - Prob. 5.82QECh. 5 - Calculate H when a 38-g sample of glucose,...Ch. 5 - Prob. 5.84QECh. 5 - The octane number of gasoline is based on a...Ch. 5 - One of the components of jet engine fuel is...Ch. 5 - Prob. 5.87QECh. 5 - Prob. 5.88QECh. 5 - When a 2.30-g sample of magnesium dissolves in...Ch. 5 - Prob. 5.90QECh. 5 - Prob. 5.91QECh. 5 - What mass of acetylene, C2H2(g), must be burned to...Ch. 5 - It takes 677 J of heat to increase the temperature...Ch. 5 - Prob. 5.94QECh. 5 - Prob. 5.96QECh. 5 - The enthalpy of combustion of liquid n-hexane,...Ch. 5 - What is Hrxn for reaction of iron(III) oxide and...Ch. 5 - Prob. 5.99QECh. 5 - Prob. 5.100QECh. 5 - In the 1880s, Frederick Trouton noted that the...Ch. 5 - Prob. 5.102QECh. 5 - Prob. 5.103QECh. 5 - Prob. 5.104QECh. 5 - Prob. 5.105QECh. 5 - A compound is 82.7% carbon and 17.3% hydrogen, and...Ch. 5 - Prob. 5.107QECh. 5 - Prob. 5.108QE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY