
Concept explainers
(a)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule does not have any chiral center, and it is not a meso compound.
Explanation of Solution
The structure of the given molecule is
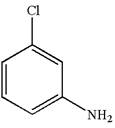
A chiral center must be an
The given molecule is determined as not a meso compound as it has no chiral center.
(b)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
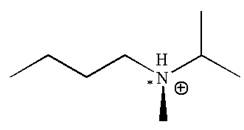
Explanation of Solution
The structure of the given molecule is
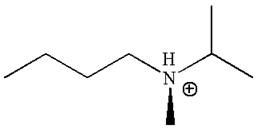
In this molecule, the nitrogen atom is a chiral center bonded to four different groups
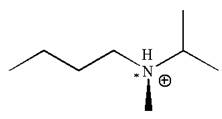
As this molecule has only one chiral center, it cannot possess any symmetry, and hence, it is not a meso compound.
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
(c)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
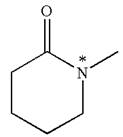
Explanation of Solution
The structure of the given molecule is
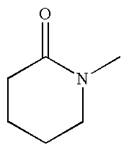
The molecule consists of a ring made up of five carbon atoms and one nitrogen atom. The nitrogen atom is bonded to three different groups having the pyramidal shape and a non-bonded electron pair pointing to the unoccupied tetrahedral corner. This makes the nitrogen a chiral center.
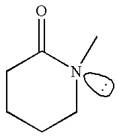
As this molecule has only one chiral center, it cannot possess any symmetry, and hence, it is not a meso compound. The chiral center is marked as
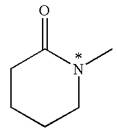
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
(d)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called a chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has no chiral center, and it is not a meso compound.
Explanation of Solution
The structure of the given molecule is
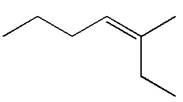
A chiral center must be an
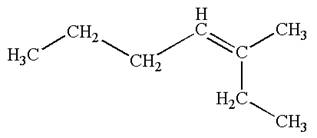
Therefore, these carbon atoms are also not chiral centers. As there are no chiral centers, the molecule is not a meso compound.
The given molecule is determined as not a meso compound as it has no chiral center.
(e)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The molecule has two chiral centers marked with
It is not a meso compound.
Explanation of Solution
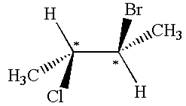
The structure of the given molecule is
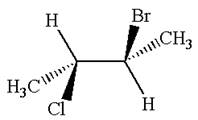
The given molecule possesses two chiral carbons. One carbon is bonded to four different groups,
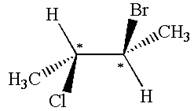
The molecule does not have symmetry plane; hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(f)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The molecule has two chiral centers marked with
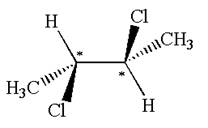
Explanation of Solution
The structure of the given molecule is
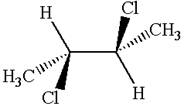
The given molecule possesses two chiral carbons bonded to four different groups,
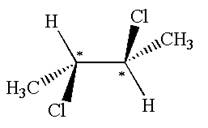
The molecule has no plane of symmetry, and hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(g)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called a chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The molecule has two chiral centers marked with
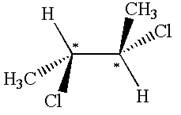
Explanation of Solution
The structure of the given molecule is
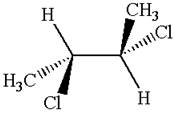
The given molecule possesses two chiral carbons bonded to four different groups,
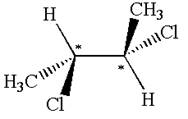
The molecule has no symmetry plane, and hence, it is not a meso compound.
The chiral centers in the given molecule are identified, and it is determined that the molecule is not a meso compound.
(h)
Interpretation:
All the chiral centers in the molecule are to be identified, and it is to be determined whether the molecule is meso.
Concept introduction:
The molecule with at least one chiral center having no plane of symmetry is called chiral molecule. A chiral center is a tetrahedral stereocenter. The atom at the chiral center must be
Answer to Problem 5.39P
The given molecule has one chiral center marked with
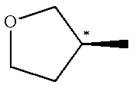
Explanation of Solution
The structure of the given molecule is
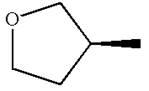
The molecule consists of a ring made up of four carbon atoms and one oxygen atom with a substituted methyl group. The carbon having the methyl substituent is a chiral center as it has four different groups bonded.
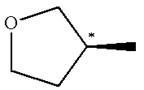
The molecule does not possess any symmetry plane; hence, it is a chiral molecule.
The chiral center in the given molecule is identified, and it is determined that the molecule is not a meso compound.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardhelp with the rf values i am so confusedarrow_forwardPredict the organic reactant of X and Y that are involved in the reaction below, and draw the skeletal ("line") structures of the missing organic reactant. Please include all steps & drawings & explanations.arrow_forward
- What are the missing reagents for the spots labeled 1 and 3? Please give a detailed explanation and include the drawings and show how the synthesis proceeds with the reagents.arrow_forwardPlease provide the complete mechanism for the reaction below and include all appropriate arrows, formal charges, and intermediates. Please draw out the answerarrow_forwardPredict the major organic product for this reaction.arrow_forward
- help me with the rf value i am so confusedarrow_forwardPredict the major organic product for this reaction.arrow_forward3) The following molecule, chloral is a common precursor to chloral hydrate, an acetal type molecule that was a first-generation anesthetic. Draw a mechanism that accounts for tis formation and speculate why it does not require the use of an acid catalyst, like most hemiacetal and acetal reaction: (10 pts) H H₂Oarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





