
Concept explainers
The amino acid
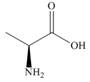
(S)-alanine
a. What is the melting point of
b. How does the melting point of a racemic mixture of
c. What is the specific rotation of
d. What is the optical rotation of a racemic mixture of
e. Label each of the following as optically active or inactive: a solution of pure
(a)
Interpretation: The melting point of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The melting point of
Explanation of Solution
The given melting point of
The melting point of
(b)
Interpretation: The melting point of a racemic mixture of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The melting point of a racemic mixture of
Explanation of Solution
The given melting point of
Therefore, the melting point of a racemic mixture of
The melting point of a racemic mixture of
(c)
Interpretation: The specific rotation of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The specific rotation of
Explanation of Solution
The specific rotation of
Thus, the specific rotation of
The specific rotation of
(d)
Interpretation: The optical rotation of a racemic mixture of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The optical rotation of a racemic mixture of
Explanation of Solution
The specific rotation of
The specific rotation of
The
Thus, the optical rotation of a racemic mixture of
The optical rotation of a racemic mixture of
(e)
Interpretation: The solutions of pure
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The pure
Explanation of Solution
The pure
A racemic mixture is obtained when
A
Thus, the pure
The pure
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry
Additional Science Textbook Solutions
SEELEY'S ANATOMY+PHYSIOLOGY
Organic Chemistry
MARINE BIOLOGY
Fundamentals of Anatomy & Physiology (11th Edition)
- What is the mechanism for this?arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward
- 3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forwardWhat is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward
- 5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forward
- Using iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forwardDraw the product formed when the following pair of compounds is treated with NaOEt in ethanol. + i CNarrow_forwardI need help with the followingarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,





