
Microbiology: An Introduction, Books a la Carte Plus Mastering Microbiology with Pearson eText -- Access Card Package (13th Edition)
13th Edition
ISBN: 9780134729336
Author: Gerard J. Tortora, Berdell R. Funke, Christine L. Case, Derek Weber, Warner Bair
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 2A
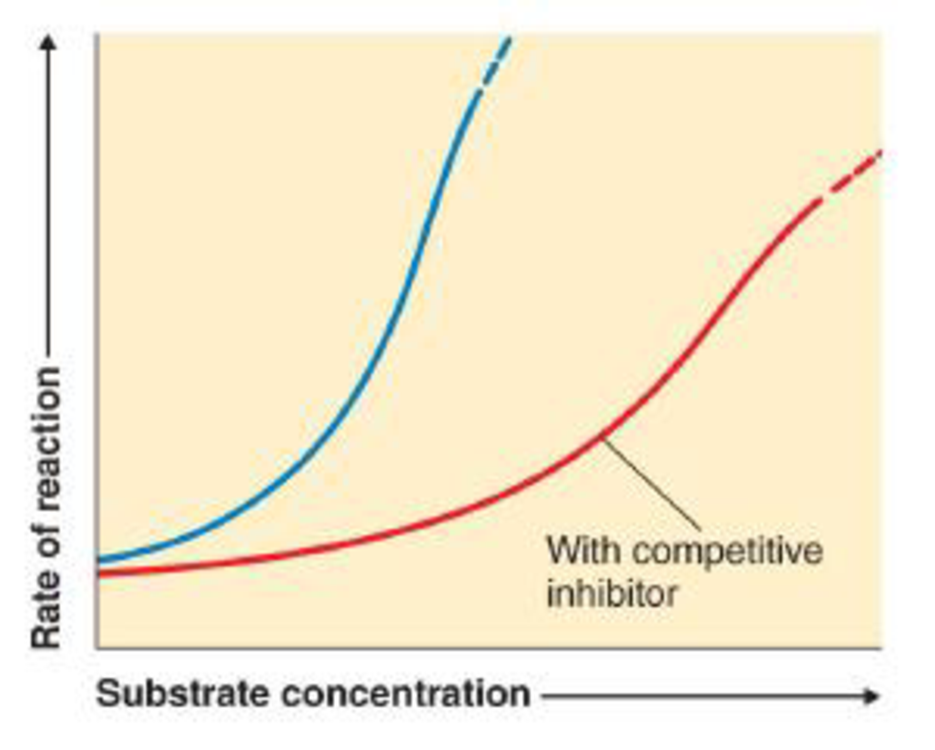
The following graph shows the normal
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
DNK dagi nukleotidlar va undan sintezlangan oqsildagi peptid boglar farqi 901 taga teng bo'lib undagi A jami H boglardan 6,5 marta kam bo'lsa DNK dagi jami H bog‘lar sonini toping
One of the ways for a cell to generate ATP is through the oxidative phosphorylation. In oxidative phosphorylation 3 ATP are produced from every one NADH molecule. In respiration, every glucose molecule produces 10 NADH molecules. If a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?
If a cell is growing on 5 glucose molecules, how much ATP can be produced using oxidative phosphorylation/aerobic respiration?
Chapter 5 Solutions
Microbiology: An Introduction, Books a la Carte Plus Mastering Microbiology with Pearson eText -- Access Card Package (13th Edition)
Ch. 5 - Prob. 1RCh. 5 - DRAW ITUsing the diagrams below, show each of the...Ch. 5 - DRAW IT An enzyme and substrate are combined. The...Ch. 5 - Define oxidation-reduction, and differentiate the...Ch. 5 - There are three mechanisms for the phosphorylation...Ch. 5 - All of the energy-producing biochemical reactions...Ch. 5 - Fill in the following table with the carbon source...Ch. 5 - Write your own definition of the chemiosmotic...Ch. 5 - Why must NADH be reoxidized? How does this happen...Ch. 5 - NAME IT What nutritional type is a colorless...
Ch. 5 - Which substance in the following reaction is being...Ch. 5 - Which of the following reactions produces the most...Ch. 5 - Prob. 3MCQCh. 5 - Which of the following compounds has the greatest...Ch. 5 - Prob. 5MCQCh. 5 - Prob. 6MCQCh. 5 - Which culture produces the most lactic acid? Use...Ch. 5 - Which culture produces the most ATP? Use the...Ch. 5 - Which culture uses NAD+? Use the following choices...Ch. 5 - Which culture uses the most glucose? Use the...Ch. 5 - Explain why, even under ideal conditions,...Ch. 5 - The following graph shows the normal rate of...Ch. 5 - Compare and contrast carbohydrate catabolism and...Ch. 5 - How much ATP could be obtained from the complete...Ch. 5 - The chemoautotroph Acidithiobacillus can obtain...Ch. 5 - Haemophilus influenzae requires hemin (X factor)...Ch. 5 - The drug Hivid, also called ddC, inhibits DNA...Ch. 5 - The bacterial enzyme streptokinase is used to...
Additional Science Textbook Solutions
Find more solutions based on key concepts
1. Suppose a chloride ion and a sodium ion are separated by a center—center distance of 5 Å. Is
the interactio...
Biochemistry: Concepts and Connections (2nd Edition)
Why do scientists think that all forms of life on earth have a common origin?
Genetics: From Genes to Genomes
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Identify the indicated cavity (Fucus). a. antheridia b. conceptacel c. receptacle d. oogonium e. none of thesearrow_forwardIdentify the indicated structure (Saprolegnia). a. antheridium O b. oospore c.sperm d. auxospore e. tetraspore Of. zygosporearrow_forwardUsing information from the primary literature (several references have been provided as a starting point below) please answer the following question: Based on your review of the literature on rewilding, what are the major scientific pros and cons for rewilding? Please note that the focus of this assignment are the (biological) scientific issues associated with rewilding. As will be discussed in class, there are a number of non-scientific issues involved or implicated in rewilding, all ultimately affecting the public acceptability of rewilding. Although these issues are important – indeed, critical – in this assignment you should focus on the biological science issues and questions. Details: You must enumerate at least two pros and at least two cons. Your answer should be no more than 500 well-chosen words, excluding references. Think carefully about how best to organize and structure your answer. Aim for high information density: say a lot, but say it succinctly. Recall Nietzche’s…arrow_forward
- Using information from the primary literature (several references have been provided as a starting point below) please answer the following question: Based on your review of the literature on rewilding, what are the major scientific pros and cons for rewilding? Please note that the focus of this assignment are the (biological) scientific issues associated with rewilding. As will be discussed in class, there are a number of non-scientific issues involved or implicated in rewilding, all ultimately affecting the public acceptability of rewilding. Although these issues are important – indeed, critical – in this assignment you should focus on the biological science issues and questions. Details: You must enumerate at least two pros and at least two cons. Your answer should be no more than 500 well-chosen words, excluding references. Think carefully about how best to organize and structure your answer. Aim for high information density: say a lot, but say it succinctly. Recall Nietzche’s…arrow_forwardNow draw a rough sketch of what the control data might look like if in addition to the specific binding, there was also a considerable amount of nonspecific binding (again using a normal dose/response curve) (do % total bound ligand vs concentration)arrow_forwardWhat are functions of cuboidal cells in the kidney? Select all that apply. Concentration of gases Dilution of chemicals Secretion of molecules Nutrition to tissues Support of tissues Absorption of moleculesarrow_forward
- question1 In plants, epithelial tissue is only found as the outermost cell layer and acts as a barrier. In humans, epithelial tissue is found inside the body as well as on the surface. What function(s) does/do epithelial tissue carry out in humans? Select all that apply. Waste storage Filtration Oxygen transport Protection Diffusion Osmosis Absorptionarrow_forwardWhat words best describes this organism? a. Unicellular/nonmotile Ob. unicellular/motile c. colonial/nonmotile d. colonial/motile e. multicelluar O f. siphonous g. none of thesearrow_forwardIdentify the phylum or class. a. Euglenophyta b. Dinoflagellata c. Bacillariophyceae d. Oomycetes e. Phaeophyceae O f. Myxomycota g. Xanthophyceae ○ h. Chrysophyceae i. Dictyosteliomycota O j. Rhodophyta Ok. Chlorophyceaens I. Charophyceaensarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning


Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning

Biology (MindTap Course List)
Biology
ISBN:9781337392938
Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. Berg
Publisher:Cengage Learning


Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License