
Explain how σ and
Interpretation:
The similarity and difference between s and p bonds is to be explained.
Concept Introduction:
- There are two types of bonds that are formed between the atoms-
- Electron in atoms occupy atomic orbitals while in molecule, electrons occupy molecular orbitals. These molecular orbitals are formed by the combination of the atomic orbitals and they can be bonding molecular orbitals and ant-bonding molecular orbitals.
- The process of mixing of atomic orbitals of similar energy to produce new molecular orbitals of equivalent energy is known as hybridization.
a) Covalent bond- the bond formed by the sharing of electrons between the atoms.
b) Ionic bond- When atoms gain/loss electrons and become ions, the electrostatic attraction between the oppositely charged ions is known as ionic bond.
Answer to Problem 1E
Similarity-They both are chemical covalent bonds and are formed by the overlapping of the atomic orbitals.
Differences-
| s- bond | ?-bond |
| Formed by the axial overlap of the atomic orbitals. | Formed by the side-ways overlap of the atomic orbitals. |
| It can be formed by the overlap of s-s, s-p or p-p orbitals. | It can only be formed by the overlap of p orbitals |
| Oriented along the internuclear axis. | Oriented perpendicular to the internuclear axis. |
| Exists independently. | Exist along with sigma bond. |
| Stronger than pi-bond. | Weaker than sigma bond. |
| More reactive. | Less reactive. |
| Determines the shape of the molecule. | Does not determine the shape of the molecule. |
Explanation of Solution
- Sigma (s) bonds- It is a type of covalent bond which is formed by the end to end overlapping of the atomic orbitals of the atoms involved in bonding.
- Pi (p)bonds -It is a type of covalent bond which is formed by the side wise overlapping of the atomic orbitals of the atoms involved in bonding.
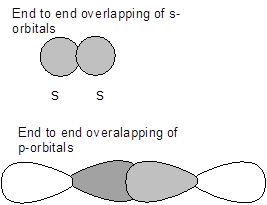

Therefore, differences between sigma and pi bonds are-
| s- bond | ?-bond |
| Formed by the axial overlap of the atomic orbitals. | Formed by the side-ways overlap of the atomic orbitals. |
| It can be formed by the overlap of s-s, s-p or p-p orbitals. | It can only be formed by the overlap of p orbitals |
| Oriented along the internuclear axis. | Oriented perpendicular to the internuclear axis. |
| Exists independently. | Exist along with sigma bond. |
| Stronger than pi-bond. | Weaker than sigma bond. |
| More reactive. | Less reactive. |
| Determines the shape of the molecule. | Does not determine the shape of the molecule. |
However, the only similarity between these two are that they both are chemical covalent bonds and are formed by the overlapping the atomic orbitals, and the main difference is that sigma bond is formed by the axial overlap of the atomic orbitals, whereas, pi bond is formed by the sideways overlapping of atomic orbitals.
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry Atoms First2e
- 35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forwardpls helparrow_forward
- 27) Draw the energy level diagram and write the full and shorthand electron configuration for a neutral sulfur atom.arrow_forwardIndicate whether these compounds are isomers, enantiomers, or tautomers. OCH OCH محمد ممدarrow_forward30) Substance A to E below are listed with several of their properties. The identities of the substances are identified in random order below: Iron, ethane, ethanol, sodium nitrate, graphite First classify each substance as either a polar covalent compound, non-polar covalent compound, ionic compound, metallic solid, or network solid. Write your predictions in the sixth coloumn of the chart, under "type of substance." Then, identify the identity of the substance in the last coloumn. Substance Melting Point Boiling Point Solubility in H₂O Electrical Conductivity Type of Substance Identity of Substance (°C) (°C) as: Solid, Liquids, Solution A -182 -88 Insoluble No/No/- B 1538 2862 Insoluble Yes/Yes/- C 308 380 Soluble Yes/Yes/Yes Ꭰ 3456 Insoluble No/-/- E -114 78 Soluble No/No/Noarrow_forward
- pls helparrow_forward28) Explain the process of galvanization. In your description, make sure to explain what metal is usually used for galvanization and why this metal used.arrow_forward29) Complete the following table Molecule H₂O NH3 Lewis Dot Diagram VSEPR Diagram Name of VSEPR Shapearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning


