
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.SE, Problem 58AP
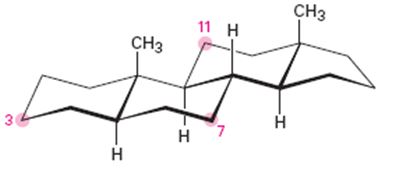
Tell whether each of the following substituents on a steroid is axial or equatorial. (A substituent that is “up” is on the top face of the molecule as drawn, and a substituent that is “down” is on the bottom face.)
(a) Substituent up at C3
(b) Substituent down at C7
(c) Substituent down at C11
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Complete the reaction
hand written please
Complete the following equations
hand written please
Complete the following equations please
hand written please
Chapter 4 Solutions
Organic Chemistry
Ch. 4.1 - Give IUPAC names for the following cycloalkanes:Ch. 4.1 - Draw structures corresponding to the following...Ch. 4.1 - Name the following cycloalkane:Ch. 4.2 - Prob. 4PCh. 4.2 - Draw the structures of the following molecules:...Ch. 4.2 - Prostaglandin F2α, a hormone that causes uterine...Ch. 4.2 - Name the following substances, including the cis-...Ch. 4.3 - Each H↔H eclipsing interaction in ethane costs...Ch. 4.3 - cis-1, 2-Dimethylcyclopropane has more strain than...Ch. 4.4 - Prob. 10P
Ch. 4.4 - Two conformations of cis-l, 3-dimethylcyclobutane...Ch. 4.6 - Draw two different chair conformations of...Ch. 4.6 - Draw two differant chair conformations of trans-1,...Ch. 4.6 - Prob. 14PCh. 4.7 - What is the energy difference between the axial...Ch. 4.7 - Prob. 16PCh. 4.7 - Look at Figure 4-12 on page 105, and estimate the...Ch. 4.8 - Draw the more stable chair conformation of the...Ch. 4.8 - Identify each substituent in the following...Ch. 4.9 - Which isomer is more stable, cis-decalin or...Ch. 4.9 - Look at the following structure of the female...Ch. 4.SE - Prob. 22VCCh. 4.SE - Name the following compound, identify each...Ch. 4.SE - A trisubstituted cyclohexane with three...Ch. 4.SE - The following cyclohexane derivative has three...Ch. 4.SE - Prob. 26VCCh. 4.SE - Draw the five cycloalkanes with the formula C5H10.Ch. 4.SE - Draw two constitutional isomers of cis-1,...Ch. 4.SE - Prob. 29APCh. 4.SE - Tell whether the following pairs of compounds are...Ch. 4.SE - Prob. 31APCh. 4.SE - Prob. 32APCh. 4.SE - Draw 1, 3, 5-trimethylcyclohexane using a hexagon...Ch. 4.SE - Hydrocortisone, a naturally occurring hormone...Ch. 4.SE - A 1, 2-cis disubstituted cyclohexane, such as...Ch. 4.SE - A 1, 2-trans disubstituted cyclohexane must have...Ch. 4.SE - Prob. 37APCh. 4.SE - Which is more stable, a 1, 4-trans disubstituted...Ch. 4.SE - cis-1, 2-Dimethylcyclobutane is less stable than...Ch. 4.SE - From the data in Figure 4-12 and Table 4-1,...Ch. 4.SE - Prob. 41APCh. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Galactose, a sugar related to glucose, contains a...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - The diaxial conformation of cis-1,...Ch. 4.SE - Approximately how much steric strain does the...Ch. 4.SE - In light of your answer to Problem 4-43, draw the...Ch. 4.SE - Prob. 50APCh. 4.SE - Prob. 51APCh. 4.SE - Using molecular models as well as structural...Ch. 4.SE - trans-Decalin is more stable than its cis isomer,...Ch. 4.SE - As mentioned in Problem 3-53, the statin drugs,...Ch. 4.SE - myo-Inositol, one of the isomers of...Ch. 4.SE - How many cis–trans stereoisomers of myo-inositol...Ch. 4.SE - The German chemist J. Bredt proposed in 1935 that...Ch. 4.SE - Tell whether each of the following substituents on...Ch. 4.SE - Prob. 59APCh. 4.SE - Prob. 60APCh. 4.SE - Ketones react with alcohols to yield products...Ch. 4.SE - Alcohols undergo an oxidation reaction to yield...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 3+ 3Cu²+ (aq) +2Al(s) → 3 Cu(s)+2A1³* (aq) 2+ Suppose the cell is prepared with 5.29 M Cu in one half-cell and 2.49 M A1³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. x10 μ ☑ 00. 18 Ar Иarrow_forwardPlease help me solve this homework problemarrow_forwardPlease help me answer this homework questionarrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. 3+ H2(g)+2OH¯ (aq) + 2Fe³+ (aq) → 2H₂O (1)+2Fe²+ (aq) 0 kJ x10 Х ? olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid An analytical chemist is titrating 184.2 mL of a 0.7800M solution of dimethylamine ((CH3) NH with a 0.3000M solution of HClO4. The pK₁ of dimethylamine is 3.27. Calculate the pH of the base solution after the chemist has added 424.1 mL of the HClO solution to it. 2 4 Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HClO 4 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ ? 000 18 Ar 1 Barrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: MnO2 (s)+4H* (aq)+2Cr²+ (aq) → Mn²+ (aq)+2H₂O (1)+2Cr³+ (aq) + 2+ 2+ 3+ Suppose the cell is prepared with 7.44 M H* and 0.485 M Cr²+ in one half-cell and 7.92 M Mn² and 3.73 M Cr³+ in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. ☐ x10 μ Х 5 ? 000 日。arrow_forward
- Calculating standard reaction free energy from standard reduction... Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Be sure your answer has the correct number of significant digits. NO (g) +H₂O (1) + Cu²+ (aq) → HNO₂ (aq) +H* (aq)+Cu* (aq) kJ - ☐ x10 x10 olo 18 Ararrow_forwardCalculating the pH of a weak base titrated with a strong acid b An analytical chemist is titrating 116.9 mL of a 0.7700M solution of aniline (C6H5NH2) with a 0.5300M solution of HNO3. The pK of aniline is 9.37. Calculate the pH of the base solution after the chemist has added 184.2 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places. pH = ☐ ☑ 5arrow_forwardQUESTION: Find the standard deviation for the 4 different groups 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5.033 4.044 334.6 268.7 4.706 3.621 305.6 234.4 4.816 3.728 340.0 262.7 4.828 4.496 304.3 283.2 4.993 3.865 244.7 143.6 STDEV = STDEV = STDEV = STDEV =arrow_forward
- QUESTION: Fill in the answers in the empty green boxes regarding 'Question 5: Calculating standard error of regression' *The images of the data showing 'coefficients for the standard curve' have been providedarrow_forwardUsing the Nernst equation to calculate nonstandard cell voltage Try Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ 2+ Sn²+ Ba(s) (aq) + Ba (s) Sn (s) + Ba²+ (aq) →>> Suppose the cell is prepared with 6.10 M Sn 2+ 2+ in one half-cell and 6.62 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 1.71 V ☐ x10 ☑ 5 0/5 ? 00. 18 Ararrow_forwardQuestion: Find both the b (gradient) and a (y-intercept) value from the list of data below: (x1 -x̄) 370.5 (y1 - ȳ) 5.240 (x2 - x̄) 142.5 (y2 - ȳ) 2.004 (x3 - x̄) 28.5 (y3 - ȳ) 0.390 (x4 - x̄) -85.5 (y4 - ȳ) -1.231 (x5 - x̄) -199.5 (y5 - ȳ) -2.829 (x6 - x̄) -256.5 (y6 - ȳ) -3.575arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License