
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.SE, Problem 31AP
Interpretation Introduction
Interpretation:
The three isomers of trans-1, 2-dichlorobutane is to be drawn.
Concept Introduction:
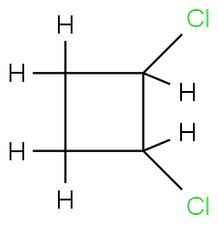
C4H6Cl2
1, 2-Dichlorocyclobutane.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Assign ALL signals for the proton and carbon NMR spectra on the following pages.
7.5
1.93
2.05
C
B
A
4
3
5
The Joh.
9
7
8
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0 ppm
9
7
8
0.86
OH 10
4
3
5
1
2
7.5
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
1.5
1.0
ppm
9
7
8
CI
4
3
5
1
2
7.0
6.5
6.0
5.5
5.0
4.5
4.0
3.5
3.0
2.5
2.0
2.21
4.00
1.5
2.00
2.07
1.0
ppm
2.76
Assign the functional group bands on the IR spectra.
Chapter 4 Solutions
Organic Chemistry
Ch. 4.1 - Give IUPAC names for the following cycloalkanes:Ch. 4.1 - Draw structures corresponding to the following...Ch. 4.1 - Name the following cycloalkane:Ch. 4.2 - Prob. 4PCh. 4.2 - Draw the structures of the following molecules:...Ch. 4.2 - Prostaglandin F2α, a hormone that causes uterine...Ch. 4.2 - Name the following substances, including the cis-...Ch. 4.3 - Each H↔H eclipsing interaction in ethane costs...Ch. 4.3 - cis-1, 2-Dimethylcyclopropane has more strain than...Ch. 4.4 - Prob. 10P
Ch. 4.4 - Two conformations of cis-l, 3-dimethylcyclobutane...Ch. 4.6 - Draw two different chair conformations of...Ch. 4.6 - Draw two differant chair conformations of trans-1,...Ch. 4.6 - Prob. 14PCh. 4.7 - What is the energy difference between the axial...Ch. 4.7 - Prob. 16PCh. 4.7 - Look at Figure 4-12 on page 105, and estimate the...Ch. 4.8 - Draw the more stable chair conformation of the...Ch. 4.8 - Identify each substituent in the following...Ch. 4.9 - Which isomer is more stable, cis-decalin or...Ch. 4.9 - Look at the following structure of the female...Ch. 4.SE - Prob. 22VCCh. 4.SE - Name the following compound, identify each...Ch. 4.SE - A trisubstituted cyclohexane with three...Ch. 4.SE - The following cyclohexane derivative has three...Ch. 4.SE - Prob. 26VCCh. 4.SE - Draw the five cycloalkanes with the formula C5H10.Ch. 4.SE - Draw two constitutional isomers of cis-1,...Ch. 4.SE - Prob. 29APCh. 4.SE - Tell whether the following pairs of compounds are...Ch. 4.SE - Prob. 31APCh. 4.SE - Prob. 32APCh. 4.SE - Draw 1, 3, 5-trimethylcyclohexane using a hexagon...Ch. 4.SE - Hydrocortisone, a naturally occurring hormone...Ch. 4.SE - A 1, 2-cis disubstituted cyclohexane, such as...Ch. 4.SE - A 1, 2-trans disubstituted cyclohexane must have...Ch. 4.SE - Prob. 37APCh. 4.SE - Which is more stable, a 1, 4-trans disubstituted...Ch. 4.SE - cis-1, 2-Dimethylcyclobutane is less stable than...Ch. 4.SE - From the data in Figure 4-12 and Table 4-1,...Ch. 4.SE - Prob. 41APCh. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Draw the two chair conformations of...Ch. 4.SE - Galactose, a sugar related to glucose, contains a...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - There are four cis-trans isomers of menthol...Ch. 4.SE - The diaxial conformation of cis-1,...Ch. 4.SE - Approximately how much steric strain does the...Ch. 4.SE - In light of your answer to Problem 4-43, draw the...Ch. 4.SE - Prob. 50APCh. 4.SE - Prob. 51APCh. 4.SE - Using molecular models as well as structural...Ch. 4.SE - trans-Decalin is more stable than its cis isomer,...Ch. 4.SE - As mentioned in Problem 3-53, the statin drugs,...Ch. 4.SE - myo-Inositol, one of the isomers of...Ch. 4.SE - How many cis–trans stereoisomers of myo-inositol...Ch. 4.SE - The German chemist J. Bredt proposed in 1935 that...Ch. 4.SE - Tell whether each of the following substituents on...Ch. 4.SE - Prob. 59APCh. 4.SE - Prob. 60APCh. 4.SE - Ketones react with alcohols to yield products...Ch. 4.SE - Alcohols undergo an oxidation reaction to yield...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Find the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forwardDraw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forward
- Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forward
- Including activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forwardCan I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forward
- Ordene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
